Repetitive mild traumatic brain injury induces ventriculomegaly and cortical thinning in juvenile rats
- PMID: 25695652
- PMCID: PMC4440238
- DOI: 10.1152/jn.00970.2014
Repetitive mild traumatic brain injury induces ventriculomegaly and cortical thinning in juvenile rats
Abstract
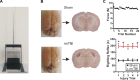
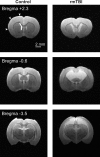
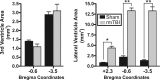
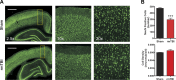
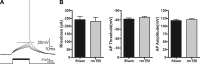
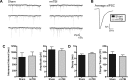
Traumatic brain injury (TBI) most frequently occurs in pediatric patients and remains a leading cause of childhood death and disability. Mild TBI (mTBI) accounts for nearly 75% of all TBI cases, yet its neuropathophysiology is still poorly understood. While even a single mTBI injury can lead to persistent deficits, repeat injuries increase the severity and duration of both acute symptoms and long-term deficits. In this study, to model pediatric repetitive mTBI (rmTBI) we subjected unrestrained juvenile animals (postnatal day 20) to repeat weight-drop impacts. Animals were anesthetized and subjected to sham injury or rmTBI once per day for 5 days. Magnetic resonance imaging (MRI) performed 14 days after injury revealed marked cortical atrophy and ventriculomegaly in rmTBI animals. Specifically, beneath the impact zone the thickness of the cortex was reduced by up to 46% and the area of the ventricles increased by up to 970%. Immunostaining with the neuron-specific marker NeuN revealed an overall loss of neurons within the motor cortex but no change in neuronal density. Examination of intrinsic and synaptic properties of layer II/III pyramidal neurons revealed no significant difference between sham-injured and rmTBI animals at rest or under convulsant challenge with the potassium channel blocker 4-aminopyridine. Overall, our findings indicate that the neuropathological changes reported after pediatric rmTBI can be effectively modeled by repeat weight drop in juvenile animals. Developing a better understanding of how rmTBI alters the pediatric brain may help improve patient care and direct "return to game" decision making in adolescents.
Keywords: MRI; concussion; electrophysiology; pediatrics; traumatic brain injury.
Copyright © 2015 the American Physiological Society.
Figures
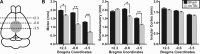



References
-
- Abdel B, Samah G, Kao HY, Kelemen E, Fenton AA, Bergold PJ. A hierarchy of neurobehavioral tasks discriminates between mild and moderate brain injury in rats. Brain Res 1280: 98–106, 2009. - PubMed
-
- Adelson PD. Animal models of traumatic brain injury in the immature: a review. Exp Toxicol Pathol 51: 130–136, 1999. - PubMed
-
- Allen GV, Gerami D, Esser MJ. Conditioning effects of repetitive mild neurotrauma on motor function in an animal model of focal brain injury. Neuroscience 99: 93–105, 2000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

