Prions--not your immunologist's pathogen
- PMID: 25695738
- PMCID: PMC4335031
- DOI: 10.1371/journal.ppat.1004624
Prions--not your immunologist's pathogen
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
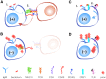
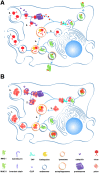
Similar articles
-
Dirty mice join the immunologist's toolkit.Microbes Infect. 2021 Jul-Aug;23(6-7):104817. doi: 10.1016/j.micinf.2021.104817. Epub 2021 Mar 27. Microbes Infect. 2021. PMID: 33785421 Review.
-
EAE: an immunologist's magic eye.Eur J Immunol. 2009 Aug;39(8):2031-5. doi: 10.1002/eji.200939568. Eur J Immunol. 2009. PMID: 19672898 Review.
-
The parasite immunologist's crystal ball.Parasite Immunol. 2006 Jun;28(6):233-4. doi: 10.1111/j.1365-3024.2006.00888.x. Parasite Immunol. 2006. PMID: 16704464 No abstract available.
-
An immunologist's vade mecum.N Y State J Med. 1957 Nov 15;57(22):3615-22. N Y State J Med. 1957. PMID: 13483906 No abstract available.
-
Primary prevention: Vaccines and the allergist-immunologist's role.Ann Allergy Asthma Immunol. 2020 Jul;125(1):1. doi: 10.1016/j.anai.2020.03.031. Ann Allergy Asthma Immunol. 2020. PMID: 32564925 Free PMC article. No abstract available.
Cited by
-
Immunization of cervidized transgenic mice with multimeric deer prion protein induces self-antibodies that antagonize chronic wasting disease infectivity in vitro.Sci Rep. 2017 Sep 5;7(1):10538. doi: 10.1038/s41598-017-11235-8. Sci Rep. 2017. PMID: 28874781 Free PMC article.
-
Perspectives on CRISPR Genome Editing to Prevent Prion Diseases in High-Risk Individuals.Biomedicines. 2024 Aug 1;12(8):1725. doi: 10.3390/biomedicines12081725. Biomedicines. 2024. PMID: 39200190 Free PMC article.
-
A Landscape Epidemiological Approach for Predicting Chronic Wasting Disease: A Case Study in Virginia, US.Front Vet Sci. 2021 Aug 24;8:698767. doi: 10.3389/fvets.2021.698767. eCollection 2021. Front Vet Sci. 2021. PMID: 34504887 Free PMC article.
-
Recombinant prion protein vaccination of transgenic elk PrP mice and reindeer overcomes self-tolerance and protects mice against chronic wasting disease.J Biol Chem. 2018 Dec 21;293(51):19812-19822. doi: 10.1074/jbc.RA118.004810. Epub 2018 Nov 5. J Biol Chem. 2018. PMID: 30397182 Free PMC article.
-
Oral vaccination as a potential strategy to manage chronic wasting disease in wild cervid populations.Front Immunol. 2023 Apr 14;14:1156451. doi: 10.3389/fimmu.2023.1156451. eCollection 2023. Front Immunol. 2023. PMID: 37122761 Free PMC article. Review.
References
-
- Prusiner SB (1982) Novel proteinaceous infectious particles cause scrapie. Science 216: 136–144. - PubMed
-
- Oesch B, Westaway D, Wälchli M, McKinley MP, Kent SBH, et al. (1985) A cellular gene encodes scrapie PrP 27–30 protein. Cell 40: 735–746. - PubMed
-
- Büeler HR, Aguzzi A, Sailer A, Greiner RA, Autenried P, et al. (1993) Mice devoid of PrP are resistant to scrapie. Cell 73: 1339–1347. Available: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgidbfrom=pubmed&id=.... - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

