Contribution of hepatic organic anion-transporting polypeptides to docetaxel uptake and clearance
- PMID: 25695959
- PMCID: PMC4394048
- DOI: 10.1158/1535-7163.MCT-14-0547
Contribution of hepatic organic anion-transporting polypeptides to docetaxel uptake and clearance
Abstract
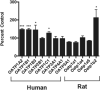
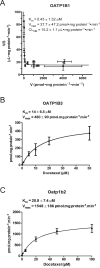
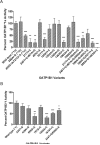
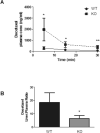
The antimicrotubular agent docetaxel is a widely used chemotherapeutic drug for the treatment of multiple solid tumors and is predominantly dependent on hepatic disposition. In this study, we evaluated drug uptake transporters capable of transporting radiolabeled docetaxel. By screening an array of drug uptake transporters in HeLa cells using a recombinant vaccinia-based method, five organic anion-transporting polypeptides (OATP) capable of docetaxel uptake were identified: OATP1A2, OATP1B1, OATP1B3, OATP1C1, and Oatp1b2. Kinetic analysis of docetaxel transport revealed similar kinetic parameters among hepatic OATP1B/1b transporters. An assessment of polymorphisms (SNPs) in SLCO1B1 and SLCO1B3 revealed that a number of OATP1B1 and OATP1B3 variants were associated with impaired docetaxel transport. A Transwell-based vectorial transport assay using MDCKII stable cells showed that docetaxel was transported significantly into the apical compartment of double-transfected (MDCKII-OATP1B1/MDR1 and MDCKII-OATP1B3/MDR1) cells compared with single-transfected (MDCKII-OATP1B1 and MDCKII-OATP1B3) cells (P < 0.05) or control (MDCKII-Co) cells (P < 0.001). In vivo docetaxel transport studies in Slco1b2(-/-) mice showed approximately >5.5-fold higher plasma concentrations (P < 0.01) and approximately 3-fold decreased liver-to-plasma ratio (P < 0.05) of docetaxel compared with wild-type (WT) mice. The plasma clearance of docetaxel in Slco1b2(-/-) mice was 83% lower than WT mice (P < 0.05). In conclusion, this study demonstrates the important roles of OATP1B transporters to the hepatic disposition and clearance of docetaxel, and supporting roles of these transporters for docetaxel pharmacokinetics.
©2015 American Association for Cancer Research.
Figures

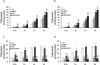
Similar articles
-
Contribution of Organic Anion-Transporting Polypeptides 1A/1B to Doxorubicin Uptake and Clearance.Mol Pharmacol. 2017 Jan;91(1):14-24. doi: 10.1124/mol.116.105544. Epub 2016 Oct 24. Mol Pharmacol. 2017. PMID: 27777271 Free PMC article.
-
Influence of polymorphic OATP1B-type carriers on the disposition of docetaxel.Clin Cancer Res. 2012 Aug 15;18(16):4433-40. doi: 10.1158/1078-0432.CCR-12-0761. Epub 2012 Jun 18. Clin Cancer Res. 2012. PMID: 22711709 Free PMC article.
-
Human OATP1B1, OATP1B3 and OATP1A2 can mediate the in vivo uptake and clearance of docetaxel.Int J Cancer. 2015 Jan 1;136(1):225-33. doi: 10.1002/ijc.28970. Epub 2014 Jun 3. Int J Cancer. 2015. PMID: 24825069
-
Role of the liver-specific transporters OATP1B1 and OATP1B3 in governing drug elimination.Expert Opin Drug Metab Toxicol. 2005 Oct;1(3):429-45. doi: 10.1517/17425255.1.3.429. Expert Opin Drug Metab Toxicol. 2005. PMID: 16863454 Review.
-
Clinical significance of organic anion transporting polypeptides (OATPs) in drug disposition: their roles in hepatic clearance and intestinal absorption.Biopharm Drug Dispos. 2013 Jan;34(1):45-78. doi: 10.1002/bdd.1823. Biopharm Drug Dispos. 2013. PMID: 23115084 Review.
Cited by
-
Key genes and molecular mechanisms related to Paclitaxel Resistance.Cancer Cell Int. 2024 Jul 13;24(1):244. doi: 10.1186/s12935-024-03415-0. Cancer Cell Int. 2024. PMID: 39003454 Free PMC article. Review.
-
Overview of organic anion transporters and organic anion transporter polypeptides and their roles in the liver.World J Clin Cases. 2019 Dec 6;7(23):3915-3933. doi: 10.12998/wjcc.v7.i23.3915. World J Clin Cases. 2019. PMID: 31832394 Free PMC article. Review.
-
Role for Drug Transporters in Chemotherapy-Induced Peripheral Neuropathy.Clin Transl Sci. 2021 Mar;14(2):460-467. doi: 10.1111/cts.12915. Epub 2020 Nov 9. Clin Transl Sci. 2021. PMID: 33142018 Free PMC article. Review.
-
Uptake Transporters of the SLC21, SLC22A, and SLC15A Families in Anticancer Therapy-Modulators of Cellular Entry or Pharmacokinetics?Cancers (Basel). 2020 Aug 12;12(8):2263. doi: 10.3390/cancers12082263. Cancers (Basel). 2020. PMID: 32806706 Free PMC article. Review.
-
Life-Threatening Docetaxel Toxicity in a Patient With Reduced-Function CYP3A Variants: A Case Report.Front Oncol. 2022 Jan 31;11:809527. doi: 10.3389/fonc.2021.809527. eCollection 2021. Front Oncol. 2022. PMID: 35174070 Free PMC article.
References
-
- Baker SD, Sparreboom A, Verweij J. Clinical pharmacokinetics of docetaxel : recent developments. Clinical pharmacokinetics. 2006;45:235–52. - PubMed
-
- Bruno R, Hille D, Riva A, Vivier N, ten Bokkel Huinnink WW, van Oosterom AT, et al. Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1998;16:187–96. - PubMed
-
- Jabir RS, Naidu R, Annuar MA, Ho GF, Munisamy M, Stanslas J. Pharmacogenetics of taxanes: impact of gene polymorphisms of drug transporters on pharmacokinetics and toxicity. Pharmacogenomics. 2012;13:1979–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

