Prevalence and distribution of VZV in temporal arteries of patients with giant cell arteritis
- PMID: 25695965
- PMCID: PMC4433460
- DOI: 10.1212/WNL.0000000000001409
Prevalence and distribution of VZV in temporal arteries of patients with giant cell arteritis
Abstract
Objective: Varicella-zoster virus (VZV) infection may trigger the inflammatory cascade that characterizes giant cell arteritis (GCA).
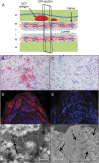
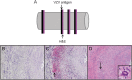
Methods: Formalin-fixed, paraffin-embedded GCA-positive temporal artery (TA) biopsies (50 sections/TA) including adjacent skeletal muscle and normal TAs obtained postmortem from subjects >50 years of age were examined by immunohistochemistry for presence and distribution of VZV antigen and by ultrastructural examination for virions. Adjacent regions were examined by hematoxylin & eosin staining. VZV antigen-positive slides were analyzed by PCR for VZV DNA.
Results: VZV antigen was found in 61/82 (74%) GCA-positive TAs compared with 1/13 (8%) normal TAs (p < 0.0001, relative risk 9.67, 95% confidence interval 1.46, 63.69). Most GCA-positive TAs contained viral antigen in skip areas. VZV antigen was present mostly in adventitia, followed by media and intima. VZV antigen was found in 12/32 (38%) skeletal muscles adjacent to VZV antigen-positive TAs. Despite formalin fixation, VZV DNA was detected in 18/45 (40%) GCA-positive VZV antigen-positive TAs, in 6/10 (60%) VZV antigen-positive skeletal muscles, and in one VZV antigen-positive normal TA. Varicella-zoster virions were found in a GCA-positive TA. In sections adjacent to those containing VZV, GCA pathology was seen in 89% of GCA-positive TAs but in none of 18 adjacent sections from normal TAs.
Conclusions: Most GCA-positive TAs contained VZV in skip areas that correlated with adjacent GCA pathology, supporting the hypothesis that VZV triggers GCA immunopathology. Antiviral treatment may confer additional benefit to patients with GCA treated with corticosteroids, although the optimal antiviral regimen remains to be determined.
© 2015 American Academy of Neurology.
Figures

Comment in
-
Varicella-zoster virus claims yet another painful scalp--Giant cell arteritis.Neurology. 2015 May 12;84(19):1918-9. doi: 10.1212/WNL.0000000000001459. Epub 2015 Feb 27. Neurology. 2015. PMID: 25724229 No abstract available.
References
-
- Lenton J, Donnelly R, Nash JR. Does temporal artery biopsy influence the management of temporal arteritis? QJM 2006;99:33–36. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
