Multifocal retinopathy in Dachshunds with CLN2 neuronal ceroid lipofuscinosis
- PMID: 25697710
- PMCID: PMC4426040
- DOI: 10.1016/j.exer.2015.02.012
Multifocal retinopathy in Dachshunds with CLN2 neuronal ceroid lipofuscinosis
Abstract
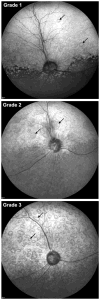
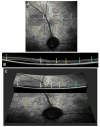
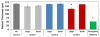
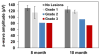
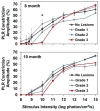
The CLN2 form of neuronal ceroid lipofuscinosis is an autosomal recessively inherited lysosomal storage disease that is characterized by progressive vision loss culminating in blindness, cognitive and motor decline, neurodegeneration, and premature death. CLN2 disease results from mutations in the gene that encodes the soluble lysosomal enzyme tripeptidyl peptidase-1. A null mutation in the TPP1 gene encoding this enzyme causes a CLN2-like disease in Dachshunds. Dachshunds that are homozygous for this mutation serve as a model for human CLN2 disease, exhibiting clinical signs and neuropathology similar to those of children with this disorder. Affected dogs reach end-stage terminal disease status at 10-11 months of age. In addition to retinal changes typical of CLN2 disease, a retinopathy consisting of multifocal, bullous retinal detachment lesions was identified in 65% of (TPP1-/-) dogs in an established research colony. These lesions did not occur in littermates that were heterozygous or homozygous for the normal TPP1 allele. Retinal changes and the functional effects of this multifocal retinopathy were examined objectively over time using ophthalmic examinations, fundus photography, electroretinography (ERG), quantitative pupillary light response (PLR) recording, fluorescein angiography, optical coherence tomography (OCT) and histopathology. The retinopathy consisted of progressive multifocal serous retinal detachments. The severity of the disease-related retinal thinning was no more serious in most detached areas than in adjacent areas of the retina that remained in close apposition to the retinal pigment epithelium. The retinopathy observed in these dogs was somewhat similar to canine multifocal retinopathy (CMR), a disease caused by a mutation of the bestrophin gene BEST1. ERG a-wave amplitudes were relatively preserved in the Dachshunds with CLN2 disease, whether or not they developed the multifocal retinopathy. The retinopathy also had minimal effects on the PLR. Histological evaluation indicated that the CLN2 disease-related retinal degeneration was not exacerbated in areas where the retina was detached except where the detached areas were very large. DNA sequence analysis ruled out a mutation in the BEST1 exons or splice junctions as a cause for the retinopathy. Perfect concordance between the TPP1 mutation and the retinopathy in the large number of dogs examined indicates that the retinopathy most likely occurs as a direct result of the TPP1 mutation. Therefore, inhibition of the development and progression of these lesions can be used as an indicator of the efficacy of therapeutic interventions currently under investigation for the treatment of CLN2 disease in the Dachshund model. In addition, these findings suggest that TPP1 mutations may underlie multifocal retinopathies of unknown cause in animals and humans.
Keywords: BEST1; Bullous retinopathy; CLN2; Canine; Dog; Neuronal ceroid lipofuscinosis; Retinal detachment; TPP1.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
Intracerebroventricular gene therapy that delays neurological disease progression is associated with selective preservation of retinal ganglion cells in a canine model of CLN2 disease.Exp Eye Res. 2016 May;146:276-282. doi: 10.1016/j.exer.2016.03.023. Epub 2016 Apr 1. Exp Eye Res. 2016. PMID: 27039708 Free PMC article.
-
Enzyme replacement therapy delays pupillary light reflex deficits in a canine model of late infantile neuronal ceroid lipofuscinosis.Exp Eye Res. 2014 Aug;125:164-72. doi: 10.1016/j.exer.2014.06.008. Epub 2014 Jun 19. Exp Eye Res. 2014. PMID: 24954537
-
Intravitreal enzyme replacement preserves retinal structure and function in canine CLN2 neuronal ceroid lipofuscinosis.Exp Eye Res. 2020 Aug;197:108130. doi: 10.1016/j.exer.2020.108130. Epub 2020 Jul 1. Exp Eye Res. 2020. PMID: 32622066 Free PMC article.
-
CLN2 Disease (Classic Late Infantile Neuronal Ceroid Lipofuscinosis).Pediatr Endocrinol Rev. 2016 Jun;13 Suppl 1:682-8. Pediatr Endocrinol Rev. 2016. PMID: 27491216 Review.
-
Enzyme Replacement Therapy in CLN2-Associated Retinopathy.Klin Monbl Augenheilkd. 2025 Mar;242(3):213-218. doi: 10.1055/a-2528-7886. Epub 2025 Mar 24. Klin Monbl Augenheilkd. 2025. PMID: 40127655 Review. English.
Cited by
-
Intracerebroventricular gene therapy that delays neurological disease progression is associated with selective preservation of retinal ganglion cells in a canine model of CLN2 disease.Exp Eye Res. 2016 May;146:276-282. doi: 10.1016/j.exer.2016.03.023. Epub 2016 Apr 1. Exp Eye Res. 2016. PMID: 27039708 Free PMC article.
-
Safety and potential efficacy of gemfibrozil as a supportive treatment for children with late infantile neuronal ceroid lipofuscinosis and other lipid storage disorders.Orphanet J Rare Dis. 2017 Jun 17;12(1):113. doi: 10.1186/s13023-017-0663-8. Orphanet J Rare Dis. 2017. PMID: 28623936 Free PMC article. Review.
-
Changes in retinal layer thickness with maturation in the dog: an in vivo spectral domain - optical coherence tomography imaging study.BMC Vet Res. 2020 Jun 30;16(1):225. doi: 10.1186/s12917-020-02390-8. BMC Vet Res. 2020. PMID: 32605619 Free PMC article.
-
Retinal Degeneration In A Mouse Model Of CLN5 Disease Is Associated With Compromised Autophagy.Sci Rep. 2017 May 9;7(1):1597. doi: 10.1038/s41598-017-01716-1. Sci Rep. 2017. PMID: 28487519 Free PMC article.
-
Intravitreal enzyme replacement inhibits progression of retinal degeneration in canine CLN2 neuronal ceroid lipofuscinosis.Exp Eye Res. 2020 Sep;198:108135. doi: 10.1016/j.exer.2020.108135. Epub 2020 Jul 4. Exp Eye Res. 2020. PMID: 32634395 Free PMC article.
References
-
- Awano T, Katz ML, O’Brien DP, Sohar I, Lobel P, Coates JR, Khan S, Johnson GC, Giger U, Johnson GS. A frame shift mutation in canine TPP1 (the ortholog of human CLN2) in a juvenile Dachshund with neuronal ceroid lipofuscinosis. Mol Genet Metab. 2006a;89:254–260. - PubMed
-
- Awano T, Katz ML, O’Brien DP, Taylor JF, Evans J, Khan S, Sohar I, Lobel P, Johnson GS. A mutation in the cathepsin D gene (CTSD) in American Bulldogs with neuronal ceroid lipofuscinosis. Mol Genet Metab. 2006b;87:341–348. - PubMed
-
- Brown KT. The electroretinogram: its components and their origins. Vision Res. 1968;8:633–677. - PubMed
-
- Ekesten B, Komaromy AM, Ofri R, Petersen-Jones SM, Narfstrom K. Guidelines for clinical electroretinography in the dog: 2012 update. Doc Ophthalmol. 2013;127:79–87. - PubMed
-
- Farias FH, Zeng R, Johnson GS, Wininger FA, Taylor JF, Schnabel RD, McKay SD, Sanders DN, Lohi H, Seppala EH, Wade CM, Lindblad-Toh K, O’Brien DP, Katz ML. A truncating mutation in ATP13A2 is responsible for adult-onset neuronal ceroid lipofuscinosis in Tibetan terriers. Neurobiol Dis. 2011;42:468–474. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

