Ligand-enabled β-C-H arylation of α-amino acids using a simple and practical auxiliary
- PMID: 25697780
- PMCID: PMC4432912
- DOI: 10.1021/ja512690x
Ligand-enabled β-C-H arylation of α-amino acids using a simple and practical auxiliary
Abstract
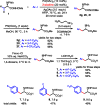
Pd-catalyzed β-C-H functionalizations of carboxylic acid derivatives using an auxiliary as a directing group have been extensively explored in the past decade. In comparison to the most widely used auxiliaries in asymmetric synthesis, the simplicity and practicality of the auxiliaries developed for C-H activation remains to be improved. We previously developed a simple N-methoxyamide auxiliary to direct β-C-H activation, albeit this system was not compatible with carboxylic acids containing α-hydrogen atoms. Herein we report the development of a pyridine-type ligand that overcomes this limitation of the N-methoxyamide auxiliary, leading to a significant improvement of β-arylation of carboxylic acid derivatives, especially α-amino acids. The arylation using this practical auxiliary is applied to the gram-scale syntheses of unnatural amino acids, bioactive molecules, and chiral bis(oxazoline) ligands.
Figures





References
-
- Evans DA, Ennis MD, Mathre DJ. J Am Chem Soc. 1982;104:1737.
- Evans DA. Aldrichim Acta. 1982;15:23.
- Myers AG, Yang BH, Chen H, McKinstry L, Kopecky DJ, Gleason JL. J Am Chem Soc. 1997;119:6496.
- Corey EJ, Enders D. Tetrahedron Lett. 1976;17:3.
- Meyers AI. Acc Chem Res. 1978;11:375.
- Job A, Janeck CF, Bettray W, Peters R, Enders D. Tetrahedron. 2002;58:2253.
-
- Giri R, Chen X, Yu J-Q. Angew Chem Int Ed. 2005;44:2112. - PubMed
- Giri R, Liang J, Lei J-G, Li J-J, Wang D-H, Chen X, Naggar IC, Guo C, Foxman BM, Yu J-Q. Angew Chem Int Ed. 2005;44:7420. - PubMed
-
For a palladium-catalyzed β-C–H oxygenation of ketones using imine directing groups, see:
- Desai LV, Hull KL, Sanford MS. J Am Chem Soc. 2004;126:9542. - PubMed
-
- Giri R, Lan Y, Liu P, Houk KN, Yu J-Q. J Am Chem Soc. 2012;134:14118. - PubMed
-
-
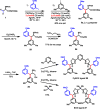
Examples of Daugulis’ bidentate 8-aminoquinoline auxiliary in C(sp3)–H activation of simple aliphatic acids using different metals. Pd:
- Zaitsev VG, Shabashov D, Daugulis O. J Am Chem Soc. 2005;127:13154. - PubMed
- Daugulis O, Do H-Q, Shabashov D. Acc Chem Res. 2009;42:1074. - PMC - PubMed
- Shabashov D, Daugulis O. J Am Chem Soc. 2010;132:3965. - PMC - PubMed
- Feng Y, Wang Y, Landgraf B, Liu S, Chen G. Org Lett. 2010;12:3414. - PubMed
-
Ni:
- Aihara Y, Chatani N. J Am Chem Soc. 2014;136:898. - PubMed
- Wu X, Zhao Y, Ge H. J Am Chem Soc. 2014;136:1789. - PubMed
- Li M, Dong J, Huang X, Li K, Wu Q, Song F, You J. Chem Commun. 2014;50:3944. - PubMed
-
Cu:
- Wang Z, Ni J, Kuninobu Y, Kanai M. Angew Chem Int Ed. 2014;53:3496. - PubMed
- Wu X, Zhao Y, Zhang G, Ge H. Angew Chem Int Ed. 2014;53:3706. - PubMed
-
Fe:
- Shang R, Ilies L, Matsumoto A, Nakamura E. J Am Chem Soc. 2013;135:6030. - PubMed
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

