Myelin basic protein associates with AβPP, Aβ1-42, and amyloid plaques in cortex of Alzheimer's disease brain
- PMID: 25697841
- PMCID: PMC4422390
- DOI: 10.3233/JAD-142013
Myelin basic protein associates with AβPP, Aβ1-42, and amyloid plaques in cortex of Alzheimer's disease brain
Abstract
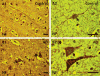
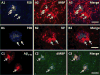
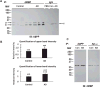
The goal of this study was to show that myelin and axons in cortical gray matter are damaged in Alzheimer's disease (AD) brain. Superior temporal gyrus gray matter of AD patients (9 male, 14 female) was compared to cognitively normal controls (8 male, 7 female). Myelin basic protein (MBP) and a degraded myelin basic protein complex (dMBP) were quantified by Western blot. Brain sections were immunostained for MBP, dMBP, axonal neurofilament protein (NF), autophagy marker microtubule-associated proteins 1A/B light chain 3B precursor (LC3B), amyloid-β protein precursor (AβPP), and amyloid markers amyloid β1-42 (Aβ1-42) and FSB. Co-immunoprecipitation and mass spectroscopy evaluated interaction of AβPP/Aβ1-42 with MBP/dMBP. Evidence of axonal injury in AD cortex included appearance of AβPP in NF stained axons, and NF at margins of amyloid plaques. Evidence of myelin injury in AD cortex included (1) increased dMBP in AD gray matter compared to control (p < 0.001); (2) dMBP in AD neurons; and (3) increased LC3B that co-localized with MBP. Evidence of interaction of AβPP/Aβ1-42 with myelin or axonal components included (1) greater binding of dMBP with AβPP in AD brain; (2) MBP at the margins of amyloid plaques; (3) dMBP co-localized with Aβ1-42 in the core of amyloid plaques in AD brains; and (4) interactions between Aβ1-42 and MBP/dMBP by co-immunoprecipitation and mass spectrometry. We conclude that damaged axons may be a source of AβPP. dMBP, MBP, and NF associate with amyloid plaques and dMBP associates with AβPP and Aβ1-42. These molecules could be involved in formation of amyloid plaques.
Keywords: Alzheimer's disease; amyloid-β; amyloid-β protein precursor; autophagy; axon damage; degraded myelin basic protein; myelin basic protein.
Figures




Similar articles
-
Inflammation Combined with Ischemia Produces Myelin Injury and Plaque-Like Aggregates of Myelin, Amyloid-β and AβPP in Adult Rat Brain.J Alzheimers Dis. 2015;46(2):507-23. doi: 10.3233/JAD-143072. J Alzheimers Dis. 2015. PMID: 25790832 Free PMC article.
-
Lipopolysaccharide Associates with Amyloid Plaques, Neurons and Oligodendrocytes in Alzheimer's Disease Brain: A Review.Front Aging Neurosci. 2018 Feb 22;10:42. doi: 10.3389/fnagi.2018.00042. eCollection 2018. Front Aging Neurosci. 2018. PMID: 29520228 Free PMC article. Review.
-
The Arctic amyloid-β precursor protein (AβPP) mutation results in distinct plaques and accumulation of N- and C-truncated Aβ.Neurobiol Aging. 2012 May;33(5):1010.e1-13. doi: 10.1016/j.neurobiolaging.2011.10.022. Epub 2011 Nov 26. Neurobiol Aging. 2012. PMID: 22118948
-
Brain amyloid-beta fragment signatures in pathological ageing and Alzheimer's disease by hybrid immunoprecipitation mass spectrometry.Neurodegener Dis. 2015;15(1):50-7. doi: 10.1159/000369465. Epub 2015 Jan 13. Neurodegener Dis. 2015. PMID: 25591542
-
Localization and Trafficking of Amyloid-β Protein Precursor and Secretases: Impact on Alzheimer's Disease.J Alzheimers Dis. 2015;45(2):329-47. doi: 10.3233/JAD-142730. J Alzheimers Dis. 2015. PMID: 25589722 Review.
Cited by
-
Study on myelin injury of AD mice treated with Shenzhiling oral liquid in the PI3K/Akt-mTOR pathway.Int J Immunopathol Pharmacol. 2020 Jan-Dec;34:2058738420923907. doi: 10.1177/2058738420923907. Int J Immunopathol Pharmacol. 2020. PMID: 32462951 Free PMC article.
-
The role of myelin damage in Alzheimer's disease pathology.Arch Med Sci. 2018 Aug 28;16(2):345-351. doi: 10.5114/aoms.2018.76863. eCollection 2020. Arch Med Sci. 2018. PMID: 32190145 Free PMC article.
-
Neuronal loss and microgliosis are restricted to the core of Aβ deposits in mouse models of Alzheimer's disease.Aging Cell. 2021 Jun;20(6):e13380. doi: 10.1111/acel.13380. Epub 2021 May 25. Aging Cell. 2021. PMID: 34080759 Free PMC article.
-
Lipopolysaccharide, Identified Using an Antibody and by PAS Staining, Is Associated With Corpora amylacea and White Matter Injury in Alzheimer's Disease and Aging Brain.Front Aging Neurosci. 2021 Nov 24;13:705594. doi: 10.3389/fnagi.2021.705594. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34899263 Free PMC article.
-
Modulation of hippocampal protein expression by a brain penetrant biologic TNF-α inhibitor in the 3xTg Alzheimer's disease mice.J Transl Med. 2024 Mar 18;22(1):291. doi: 10.1186/s12967-024-05008-x. J Transl Med. 2024. PMID: 38500108 Free PMC article.
References
-
- Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. - PubMed
-
- Alzheimer A, Forstl H, Levy R. On certain peculiar diseases of old age. Hist Psychiatry. 1991;2:71–101. - PubMed
-
- Moller HJ, Graeber MB. The case described by Alois Alzheimer in 1911. Historical and conceptual perspectives based on the clinical record and neurohistological sections. Eur Arch Psychiatry Clin Neurosci. 1998;248:111–122. - PubMed
-
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

