Local Change in Urinary Bladder Contractility Following CNS Dopamine Denervation in the 6-OHDA Rat Model of Parkinson's Disease
- PMID: 25697958
- PMCID: PMC4923752
- DOI: 10.3233/JPD-140509
Local Change in Urinary Bladder Contractility Following CNS Dopamine Denervation in the 6-OHDA Rat Model of Parkinson's Disease
Abstract
Background: Urinary problems, including urinary frequency, urgency, and nocturia are some of the non-motor symptoms that correlate most with poor quality of life in Parkinson's disease. However, the mechanism behind these symptoms is poorly understood, in particular regarding peripheral bladder pathophysiology following dopamine degeneration.
Objective: In this study, we compared the contractile responsiveness of urinary bladder from the 6-OHDA unilateral rat model of Parkinson's disease with that of normal untreated animals.
Methods: The contractility of the urinary detrusor muscle was evaluated in bladder strip preparations using electrical field stimulation, and muscarinic and purinoceptor stimulations in an vitro organ bath setup.
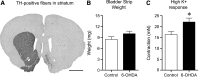
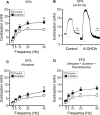
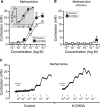
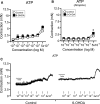
Results: Our data show that the overall contractile response following electrical field stimulation was significantly higher (43% at maximum contraction by 20-40 Hz stimulation) in the 6-OHDA-lesioned rats as compared to control animals. This increase was associated with a significant increase in the cholinergic contractile response, where the muscarinic agonist methacholine produced a 44% (at 10 -4 M concentration) higher response in the 6-OHDA-treated rats as compared to controls with a significant left-shift of the dose response. This indicates an altered sensitivity of the muscarinic receptor system following the specific central 6-OHDA-induced dopamine depletion. In addition a 36% larger contraction of strips from the 6-OHDA animals was also observed with purinoceptor activation using the agonist ATP (5×10 -3 M) during atropine treatment.
Conclusions: Our data shows that it is not only the central dopamine control of the micturition reflex that is altered in Parkinson's disease, but also the local contractile function of the urinary bladder. The current study draws attention to a mechanism of urinary dysfunction in Parkinson's disease that has previously not been described.
Keywords: Urinary bladder pathophysiology; detrusor muscle; muscarinic receptor; parasympathetic nervous system.
Figures
Similar articles
-
Berberine improves neurogenic contractile response of bladder detrusor muscle in streptozotocin-induced diabetic rats.J Ethnopharmacol. 2013 Dec 12;150(3):1128-36. doi: 10.1016/j.jep.2013.10.039. Epub 2013 Oct 30. J Ethnopharmacol. 2013. PMID: 24184080
-
Alterations in nerve-evoked bladder contractions in a coronavirus-induced mouse model of multiple sclerosis.PLoS One. 2014 Oct 13;9(10):e109314. doi: 10.1371/journal.pone.0109314. eCollection 2014. PLoS One. 2014. PMID: 25310403 Free PMC article.
-
A novel in situ urinary bladder model for studying afferent and efferent mechanisms in the micturition reflex in the rat.Neurourol Urodyn. 2014 Jun;33(5):550-7. doi: 10.1002/nau.22435. Epub 2013 May 29. Neurourol Urodyn. 2014. PMID: 23720131
-
Contractility of urinary bladder and vas deferens after sensory denervation by capsaicin treatment of newborn rats.Br J Pharmacol. 1995 Jan;114(1):166-70. doi: 10.1111/j.1476-5381.1995.tb14921.x. Br J Pharmacol. 1995. PMID: 7712013 Free PMC article.
-
On the functional role of muscarinic M2 receptors in cholinergic and purinergic responses in the rat urinary bladder.Eur J Pharmacol. 2001 Oct 12;428(3):357-64. doi: 10.1016/s0014-2999(01)01286-9. Eur J Pharmacol. 2001. PMID: 11689195
Cited by
-
6-OHDA-Induced Changes in Colonic Segment Contractility in the Rat Model of Parkinson's Disease.Gastroenterol Res Pract. 2023 Jan 27;2023:9090524. doi: 10.1155/2023/9090524. eCollection 2023. Gastroenterol Res Pract. 2023. PMID: 36743531 Free PMC article.
-
Animal models of Parkinson's disease: a guide to selecting the optimal model for your research.Neuronal Signal. 2021 Dec 8;5(4):NS20210026. doi: 10.1042/NS20210026. eCollection 2021 Dec. Neuronal Signal. 2021. PMID: 34956652 Free PMC article. Review.
-
Desipramine, commonly used as a noradrenergic neuroprotectant in 6-OHDA-lesions, leads to local functional changes in the urinary bladder and gastrointestinal tract in healthy rats.Heliyon. 2020 Nov 16;6(11):e05472. doi: 10.1016/j.heliyon.2020.e05472. eCollection 2020 Nov. Heliyon. 2020. PMID: 33251357 Free PMC article.
-
Therapeutic Approaches to Non-Motor Symptoms of Parkinson's Disease: A Current Update on Preclinical Evidence.Curr Neuropharmacol. 2023;21(3):560-577. doi: 10.2174/1570159X20666221005090126. Curr Neuropharmacol. 2023. PMID: 36200159 Free PMC article.
-
Animal Model for Lower Urinary Tract Dysfunction in Parkinson's Disease.Int J Mol Sci. 2020 Sep 7;21(18):6520. doi: 10.3390/ijms21186520. Int J Mol Sci. 2020. PMID: 32906613 Free PMC article. Review.
References
-
- Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, Bottacchi E, Cannas A, Ceravolo G, Ceravolo R, Cicarelli G, Gaglio RM, Giglia RM, Iemolo F, Manfredi M, Meco G, Nicoletti A, Pederzoli M, Petrone A, Pisani A, Pontieri FE, Quatrale R, Ramat S, Scala R, Volpe G, Zappulla S, Bentivoglio AR, Stocchi F, Trianni G, Dotto PD, group Ps. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord. 2009;24:1641–1649. - PubMed
-
- Gallagher DA, Lees AJ, Schrag A. What are the most important nonmotor symptoms in patients with Parkinson’s disease and are we missing them? Mov Disord. 2010;25:2493–2500. - PubMed
-
- Martinez-Martin P. The importance of non-motor disturbances to quality of life in Parkinson’s disease. J Neurol Sci. 2011;310:12–16. - PubMed
-
- Winge K, Fowler CJ. Bladder dysfunction in Parkinsonism: Mechanisms, prevalence, symptoms, and management. Mov Disord. 2006;21:737–745. - PubMed
-
- Winge K, Skau AM, Stimpel H, Nielsen KK, Werdelin L. Prevalence of bladder dysfunction in Parkinsons disease. Neurourol Urodyn. 2006;25:116–122. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

