Long-term Safety and Antitumor Activity in the Phase 1-2 Study of Enzalutamide in Pre- and Post-docetaxel Castration-Resistant Prostate Cancer
- PMID: 25698064
- PMCID: PMC5013546
- DOI: 10.1016/j.eururo.2015.01.026
Long-term Safety and Antitumor Activity in the Phase 1-2 Study of Enzalutamide in Pre- and Post-docetaxel Castration-Resistant Prostate Cancer
Abstract
Background: Given that some patients with castration-resistant prostate cancer (CRPC) have shown extended responses to the androgen receptor inhibitor enzalutamide, long-term safety of this drug is of interest.
Objective: To evaluate the long-term safety and antitumor activity of enzalutamide in CRPC patients.
Design, setting, and participants: This phase 1-2 study evaluated enzalutamide in 140 CRPC patients with and without prior chemotherapy. Initial findings were published in 2010. We report updated results from an additional 17-mo follow-up for antitumor activity and >4 yr for safety.
Intervention: Patients received 30-600mg/d oral enzalutamide. During long-term dosing, all patients were switched first to the maximum tolerated dose of 240mg/d and then to the phase 3 dose of 160mg/d.
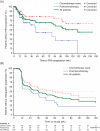
Outcome measurements and statistical analysis: Safety was assessed regularly. The Kaplan-Meier method was used to estimate the distributions of time to prostate-specific antigen (PSA) progression and time to radiographic progression.
Results and limitations: The safety profile of enzalutamide was consistent over time, with little change in the rates of commonly reported adverse events (AEs) or the incidence of grade 3/4 AEs. Fatigue of any grade was the most common dose-dependent AE, experienced by 70% of patients, with 14% of patients reporting grade 3/4 fatigue. The median time to PSA progression was not reached for chemotherapy-naive patients and was 45 wk for postchemotherapy patients; the corresponding median time to radiographic progression was 56 wk and 25 wk.
Conclusions: Enzalutamide showed durable antitumor activity in chemotherapy-naive and postchemotherapy patients, and was well tolerated, even in patients treated for 4 yr.
Patient summary: Enzalutamide was active against prostate cancer and was well tolerated, even for up to 4 yr of treatment, supporting its potential for long-term use in men with prostate cancer. Fatigue was the most common side effect, occurring at varying degrees of severity in most patients.
Keywords: Androgen receptor inhibitor; Castration-resistant prostate cancer; Enzalutamide; Long-term follow-up; MDV3100; Tolerability.
Copyright © 2015. Published by Elsevier B.V.
Figures
Comment in
-
Long-term Efficacy and Safety Results: Can Enzalutamide Challenge the Dogma of Androgen Deprivation Therapy in Hormone-naïve Prostate Cancer?Eur Urol. 2015 Nov;68(5):802-4. doi: 10.1016/j.eururo.2015.04.019. Epub 2015 Apr 24. Eur Urol. 2015. PMID: 25922192 No abstract available.
References
-
- Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–61. - PubMed
-
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous