Modelling the vascular response to sympathetic postganglionic nerve activity
- PMID: 25698230
- PMCID: PMC4386929
- DOI: 10.1016/j.jtbi.2015.01.037
Modelling the vascular response to sympathetic postganglionic nerve activity
Abstract
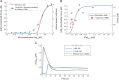
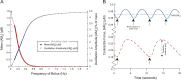
This paper explores the influence of burst properties of the sympathetic nervous system on arterial contractility. Specifically, a mathematical model is constructed of the pathway from action potential generation in a sympathetic postganglionic neurone to contraction of an arterial smooth muscle cell. The differential equation model is a synthesis of models of the individual physiological processes, and is shown to be consistent with physiological data. The model is found to be unresponsive to tonic (regular) stimulation at typical frequencies recorded in sympathetic efferents. However, when stimulated at the same average frequency, but with repetitive respiratory-modulated burst patterns, it produces marked contractions. Moreover, the contractile force produced is found to be highly dependent on the number of spikes in each burst. In particular, when the model is driven by preganglionic spike trains recorded from wild-type and spontaneously hypertensive rats (which have increased spiking during each burst) the contractile force was found to be 10-fold greater in the hypertensive case. An explanation is provided in terms of the summative increased release of noradrenaline. Furthermore, the results suggest the marked effect that hypertensive spike trains had on smooth muscle cell tone can provide a significant contribution to the pathology of hypertension.
Keywords: Bursting; Calcium dynamics; Hypertension; Neurone dynamics; Sympathetic nervous system.
Copyright © 2015 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures






References
-
- Bao J.X., Gonon F., Stjarne L. Kinetics of ATP- and noradrenaline-mediated sympathetic neuromuscular transmission in rat tail artery. Acta Physiol. Scand. 1993;149(December (4)):503–519. - PubMed
-
- Bennett M.R., Farnell L., Gibson W.G. A quantitative description of the contraction of blood vessels following the release of noradrenaline from sympathetic varicosities. J. Theor. Biol. 2005;234(May (1)):107–122. - PubMed
-
- Burnstock G. Purinergic cotransmission. Exp. Physiol. 2009;94(January (1)):20–24. - PubMed
-
- Carnevale N.T., Hines M.L. Cambridge University Press; Cambridge, UK: 2006. The NEURON Book.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

