Protein kinase D1 drives pancreatic acinar cell reprogramming and progression to intraepithelial neoplasia
- PMID: 25698580
- PMCID: PMC4394184
- DOI: 10.1038/ncomms7200
Protein kinase D1 drives pancreatic acinar cell reprogramming and progression to intraepithelial neoplasia
Abstract
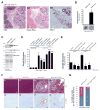
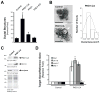
The transdifferentiation of pancreatic acinar cells to a ductal phenotype (acinar-to-ductal metaplasia, ADM) occurs after injury or inflammation of the pancreas and is a reversible process. However, in the presence of activating Kras mutations or persistent epidermal growth factor receptor (EGF-R) signalling, cells that underwent ADM can progress to pancreatic intraepithelial neoplasia (PanIN) and eventually pancreatic cancer. In transgenic animal models, ADM and PanINs are initiated by high-affinity ligands for EGF-R or activating Kras mutations, but the underlying signalling mechanisms are not well understood. Here, using a conditional knockout approach, we show that protein kinase D1 (PKD1) is sufficient to drive the reprogramming process to a ductal phenotype and progression to PanINs. Moreover, using 3D explant culture of primary pancreatic acinar cells, we show that PKD1 acts downstream of TGFα and Kras, to mediate formation of ductal structures through activation of the Notch pathway.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Comment in
-
Pancreatic oncogenic signaling cascades converge at Protein Kinase D1.Cell Cycle. 2015;14(10):1489-90. doi: 10.1080/15384101.2015.1032646. Cell Cycle. 2015. PMID: 25928263 Free PMC article. No abstract available.
References
-
- Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897–909. - PubMed
-
- Hruban RH, Wilentz RE, Goggins M, Offerhaus GJ, Yeo CJ, Kern SE. Pathology of incipient pancreatic cancer. Ann Oncol. 1999;10 (Suppl 4):9–11. - PubMed
-
- Korc M. Role of growth factors in pancreatic cancer. Surg Oncol Clin N Am. 1998;7:25–41. - PubMed
-
- Hruban RH, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–586. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA136754/CA/NCI NIH HHS/United States
- R21 CA135102/CA/NCI NIH HHS/United States
- R01 CA140290/CA/NCI NIH HHS/United States
- R01 CA081436/CA/NCI NIH HHS/United States
- P50CA102701/CA/NCI NIH HHS/United States
- R01 CA142580/CA/NCI NIH HHS/United States
- CA142580/CA/NCI NIH HHS/United States
- CA135102,/CA/NCI NIH HHS/United States
- R01 CA136754/CA/NCI NIH HHS/United States
- CA122086/CA/NCI NIH HHS/United States
- CA140290/CA/NCI NIH HHS/United States
- R01 CA140182/CA/NCI NIH HHS/United States
- P50 CA102701/CA/NCI NIH HHS/United States
- R01 CA129127/CA/NCI NIH HHS/United States
- R01 CA159222/CA/NCI NIH HHS/United States
- CA081436/CA/NCI NIH HHS/United States
- CA140182/CA/NCI NIH HHS/United States
- R01 GM086435/GM/NIGMS NIH HHS/United States
- GM86435/GM/NIGMS NIH HHS/United States
- R01 CA122086/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

