Origin, evolution and innate immune control of simian foamy viruses in humans
- PMID: 25698621
- PMCID: PMC7185842
- DOI: 10.1016/j.coviro.2014.12.003
Origin, evolution and innate immune control of simian foamy viruses in humans
Abstract
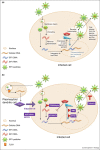
Most viral pathogens that have emerged in humans have originated from various animal species. Emergence is a multistep process involving an initial spill-over of the infectious agent into single individuals and its subsequent dissemination into the human population. Similar to simian immunodeficiency viruses and simian T lymphotropic viruses, simian foamy viruses (SFV) are retroviruses that are widespread among non-human primates and can be transmitted to humans, giving rise to a persistent infection, which seems to be controlled in the case of SFV. In this review, we present current data on the discovery, cross-species transmission, and molecular evolution of SFV in human populations initially infected and thus at risk for zoonotic emergence.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures


References
-
- Locatelli S., Peeters M. Cross-species transmission of simian retroviruses: how and why they could lead to the emergence of new diseases in the human population. AIDS. 2012;26:659–673. - PubMed
-
- Enders J.F., Peebles T.C. Propagation in tissue cultures of cytopathogenic agents from patients with measles. Proc Soc Exp Biol Med. 1954;86:277–286. - PubMed
-
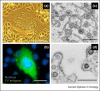
- Murray S.M., Picker L.J., Axthelm M.K., Hudkins K., Alpers C.E., Linial M.L. Replication in a superficial epithelial cell niche explains the lack of pathogenicity of primate foamy virus infections. J Virol. 2008;82:5981–5985. - PMC - PubMed
-
This study provides convincing evidence of SFV replication in the oral cavity of SFV-infected animals. This is a major argument in favor of the special mode of transmission of this retrovirus, mainly by bites, as supported by epidemiological data.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

