The MAPK and PI3K pathways mediate CNTF-induced neuronal survival and process outgrowth in hypothalamic organotypic cultures
- PMID: 25698661
- PMCID: PMC4580676
- DOI: 10.1007/s12079-015-0268-8
The MAPK and PI3K pathways mediate CNTF-induced neuronal survival and process outgrowth in hypothalamic organotypic cultures
Abstract
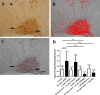
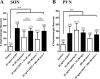
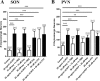
While collateral sprouting has been shown to occur in a variety of neuronal populations, the factor or factors responsible for mediating the sprouting response remain largely un-defined. There is evidence indicating that ciliary neurotrophic factor (CNTF) may play an important role in promoting neuronal survival and process outgrowth in neuronal phenotypes tested to date. We previously demonstrated that the astrocytic Jak-STAT pathway is necessary to mediate CNTF-induced oxytocinergic (OT) neuronal survival; however, the mechanism (s) of CNTF-mediated process outgrowth remain unknown. Our working hypothesis is that CNTF mediates differential neuroprotective responses via different intracellular signal transduction pathways. In order to test this hypothesis, we utilized stationary hypothalamic organotypic cultures to assess the contribution of the MAPK-ERK and PI3-AKT pathways to OT neuron survival and process outgrowth. Our results demonstrate that the MAPK-ERK½ pathway mediates CNTF-induced neuronal survival. Moreover, we show that inhibition of the p38-, JNK-MAPK, and mTOR pathways prevents loss OT neurons following axotomy. We also provide quantitative evidence indicating that CNTF promotes process outgrowth of OT neurons via the PI3K-AKT pathway. Together, these data indicate that distinct intracellular signaling pathways mediate diverse neuroprotective processes in response to CNTF.
Keywords: AKT; Axonal regeneration; CNTF; MAPK; NFkB; Neuronal survival; Organotypic culture; PI3K; STAT3.
Figures



References
-
- Askvig JM, Lo DY, Sudbeck AW, Behm KE, Leiphon LJ, Watt JA. Inhibition of the Jak-STAT pathway prevents CNTF-mediated survival of axotomized oxytocinergic magnocellular neurons in organotypic cultures of the rat supraoptic nucleus. Exp Neurol. 2013;240:75–87. doi: 10.1016/j.expneurol.2012.10.023. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

