Behavior-linked FoxP2 regulation enables zebra finch vocal learning
- PMID: 25698728
- PMCID: PMC4331621
- DOI: 10.1523/JNEUROSCI.3715-14.2015
Behavior-linked FoxP2 regulation enables zebra finch vocal learning
Abstract
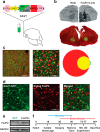
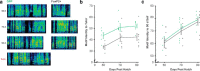
Mutations in the FOXP2 transcription factor cause an inherited speech and language disorder, but how FoxP2 contributes to learning of these vocal communication signals remains unclear. FoxP2 is enriched in corticostriatal circuits of both human and songbird brains. Experimental knockdown of this enrichment in song control neurons of the zebra finch basal ganglia impairs tutor song imitation, indicating that adequate FoxP2 levels are necessary for normal vocal learning. In unmanipulated birds, vocal practice acutely downregulates FoxP2, leading to increased vocal variability and dynamic regulation of FoxP2 target genes. To determine whether this behavioral regulation is important for song learning, here, we used viral-driven overexpression of FoxP2 to counteract its downregulation. This manipulation disrupted the acute effects of song practice on vocal variability and caused inaccurate song imitation. Together, these findings indicate that dynamic behavior-linked regulation of FoxP2, rather than absolute levels, is critical for vocal learning.
Keywords: basal ganglia; birdsong; forkhead; language; procedural learning; speech.
Copyright © 2015 the authors 0270-6474/15/352885-10$15.00/0.
Figures



References
-
- Böhner J. Early acquisition of song in the zebra finch, Taeniopygia guttata. Anim Behav. 1990;39:369–374. doi: 10.1016/S0003-3472(05)80883-8. - DOI
-
- Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, Reier PJ, Mandel RJ, Muzyczka N. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10:302–317. doi: 10.1016/j.ymthe.2004.05.024. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
