Cell assemblies of the basal forebrain
- PMID: 25698736
- PMCID: PMC6605588
- DOI: 10.1523/JNEUROSCI.4432-14.2015
Cell assemblies of the basal forebrain
Abstract
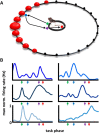
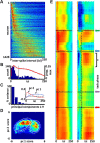
The basal forebrain comprises several heterogeneous neuronal subgroupings having modular projection patterns to discrete sets of cortical subregions. Each cortical region forms recurrent projections, via prefrontal cortex, that reach the specific basal forebrain subgroups from which they receive afferents. This architecture enables the basal forebrain to selectively modulate cortical responsiveness according to current processing demands. Theoretically, optimal functioning of this distributed network would be enhanced by temporal coordination among coactive basal forebrain neurons, or the emergence of "cell assemblies." The present work demonstrates assembly formation in rat basal forebrain neuronal populations during a selective attention task. Neuron pairs exhibited coactivation patterns organized within beta-frequency time windows (55 ms), regardless of their membership within distinct bursting versus nonbursting basal forebrain subpopulations. Thus, the results reveal a specific temporal framework for integration of information within basal forebrain networks and for the modulation of cortical responsiveness.
Keywords: assembly; beta; corticopetal; generalized linear model.
Copyright © 2015 the authors 0270-6474/15/352992-09$15.00/0.
Figures


Similar articles
-
Distinct Temporal Coordination of Spontaneous Population Activity between Basal Forebrain and Auditory Cortex.Front Neural Circuits. 2017 Sep 14;11:64. doi: 10.3389/fncir.2017.00064. eCollection 2017. Front Neural Circuits. 2017. PMID: 28959191 Free PMC article.
-
Basal Forebrain Gating by Somatostatin Neurons Drives Prefrontal Cortical Activity.Cereb Cortex. 2019 Jan 1;29(1):42-53. doi: 10.1093/cercor/bhx302. Cereb Cortex. 2019. PMID: 29161383
-
Visual training paired with electrical stimulation of the basal forebrain improves orientation-selective visual acuity in the rat.Brain Struct Funct. 2014 Jul;219(4):1493-507. doi: 10.1007/s00429-013-0582-y. Epub 2013 May 23. Brain Struct Funct. 2014. PMID: 23700106
-
The basal forebrain corticopetal system revisited.Ann N Y Acad Sci. 1999 Jun 29;877:339-67. doi: 10.1111/j.1749-6632.1999.tb09276.x. Ann N Y Acad Sci. 1999. PMID: 10415658 Review.
-
Optogenetic Dissection of the Basal Forebrain Neuromodulatory Control of Cortical Activation, Plasticity, and Cognition.J Neurosci. 2015 Oct 14;35(41):13896-903. doi: 10.1523/JNEUROSCI.2590-15.2015. J Neurosci. 2015. PMID: 26468190 Free PMC article. Review.
Cited by
-
Specific Basal Forebrain-Cortical Cholinergic Circuits Coordinate Cognitive Operations.J Neurosci. 2018 Oct 31;38(44):9446-9458. doi: 10.1523/JNEUROSCI.1676-18.2018. J Neurosci. 2018. PMID: 30381436 Free PMC article. Review.
-
Differential recruitment of ventral pallidal e-types by behaviorally salient stimuli during Pavlovian conditioning.iScience. 2021 Mar 31;24(4):102377. doi: 10.1016/j.isci.2021.102377. eCollection 2021 Apr 23. iScience. 2021. PMID: 33912818 Free PMC article.
-
Phase precession in the human hippocampus and entorhinal cortex.Cell. 2021 Jun 10;184(12):3242-3255.e10. doi: 10.1016/j.cell.2021.04.017. Epub 2021 May 11. Cell. 2021. PMID: 33979655 Free PMC article.
-
Multiplexed oscillations and phase rate coding in the basal forebrain.Sci Adv. 2018 Aug 1;4(8):eaar3230. doi: 10.1126/sciadv.aar3230. eCollection 2018 Aug. Sci Adv. 2018. PMID: 30083600 Free PMC article.
-
Distinct Temporal Coordination of Spontaneous Population Activity between Basal Forebrain and Auditory Cortex.Front Neural Circuits. 2017 Sep 14;11:64. doi: 10.3389/fncir.2017.00064. eCollection 2017. Front Neural Circuits. 2017. PMID: 28959191 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
