7 tesla FMRI reveals systematic functional organization for binocular disparity in dorsal visual cortex
- PMID: 25698743
- PMCID: PMC4331627
- DOI: 10.1523/JNEUROSCI.3047-14.2015
7 tesla FMRI reveals systematic functional organization for binocular disparity in dorsal visual cortex
Abstract
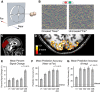
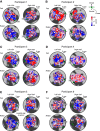
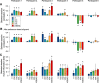
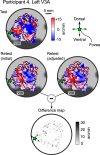
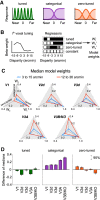
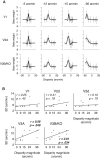
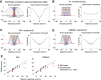
The binocular disparity between the views of the world registered by the left and right eyes provides a powerful signal about the depth structure of the environment. Despite increasing knowledge of the cortical areas that process disparity from animal models, comparatively little is known about the local architecture of stereoscopic processing in the human brain. Here, we take advantage of the high spatial specificity and image contrast offered by 7 tesla fMRI to test for systematic organization of disparity representations in the human brain. Participants viewed random dot stereogram stimuli depicting different depth positions while we recorded fMRI responses from dorsomedial visual cortex. We repeated measurements across three separate imaging sessions. Using a series of computational modeling approaches, we report three main advances in understanding disparity organization in the human brain. First, we show that disparity preferences are clustered and that this organization persists across imaging sessions, particularly in area V3A. Second, we observe differences between the local distribution of voxel responses in early and dorsomedial visual areas, suggesting different cortical organization. Third, using modeling of voxel responses, we show that higher dorsal areas (V3A, V3B/KO) have properties that are characteristic of human depth judgments: a simple model that uses tuning parameters estimated from fMRI data captures known variations in human psychophysical performance. Together, these findings indicate that human dorsal visual cortex contains selective cortical structures for disparity that may support the neural computations that underlie depth perception.
Keywords: V3A; binocular disparity; fMRI; psychophysics; ultra-high-field imaging; visual cortex.
Copyright © 2015 Goncalves et al.
Figures




Similar articles
-
Ultra-High-Field Neuroimaging Reveals Fine-Scale Processing for 3D Perception.J Neurosci. 2021 Oct 6;41(40):8362-8374. doi: 10.1523/JNEUROSCI.0065-21.2021. Epub 2021 Aug 19. J Neurosci. 2021. PMID: 34413206 Free PMC article.
-
Stereoscopic processing of crossed and uncrossed disparities in the human visual cortex.BMC Neurosci. 2017 Dec 21;18(1):80. doi: 10.1186/s12868-017-0395-7. BMC Neurosci. 2017. PMID: 29268696 Free PMC article.
-
Characterizing Human Disparity Tuning Properties Using Population Receptive Field Mapping.J Neurosci. 2025 Mar 19;45(12):e0795242025. doi: 10.1523/JNEUROSCI.0795-24.2025. J Neurosci. 2025. PMID: 39919834 Free PMC article.
-
Human cortical areas underlying the perception of optic flow: brain imaging studies.Int Rev Neurobiol. 2000;44:269-92. doi: 10.1016/s0074-7742(08)60746-1. Int Rev Neurobiol. 2000. PMID: 10605650 Review.
-
The processing of stereoscopic information in human visual cortex: psychophysical and electrophysiological evidence.Clin Electroencephalogr. 2001 Jul;32(3):152-9. doi: 10.1177/155005940103200310. Clin Electroencephalogr. 2001. PMID: 11512379 Review.
Cited by
-
Mapping Functional Topography in the Macaque Ventral Visual Pathway.J Neurosci. 2015 Aug 12;35(32):11171-3. doi: 10.1523/JNEUROSCI.2108-15.2015. J Neurosci. 2015. PMID: 26269627 Free PMC article. No abstract available.
-
High resolution data analysis strategies for mesoscale human functional MRI at 7 and 9.4T.Neuroimage. 2018 Jan 1;164:48-58. doi: 10.1016/j.neuroimage.2017.03.058. Epub 2017 Apr 14. Neuroimage. 2018. PMID: 28416453 Free PMC article.
-
The visual white matter: The application of diffusion MRI and fiber tractography to vision science.J Vis. 2017 Feb 1;17(2):4. doi: 10.1167/17.2.4. J Vis. 2017. PMID: 28196374 Free PMC article. Review.
-
Feedback from human posterior parietal cortex enables visuospatial category representations as early as primary visual cortex.Brain Behav. 2017 Dec 18;8(1):e00886. doi: 10.1002/brb3.886. eCollection 2018 Jan. Brain Behav. 2017. PMID: 29568684 Free PMC article.
-
Unique Neural Activity Patterns Among Lower Order Cortices and Shared Patterns Among Higher Order Cortices During Processing of Similar Shapes With Different Stimulus Types.Iperception. 2021 May 26;12(3):20416695211018222. doi: 10.1177/20416695211018222. eCollection 2021 May-Jun. Iperception. 2021. PMID: 34104383 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
