Cell-specific activity-dependent fractionation of layer 2/3→5B excitatory signaling in mouse auditory cortex
- PMID: 25698747
- PMCID: PMC4331630
- DOI: 10.1523/JNEUROSCI.0836-14.2015
Cell-specific activity-dependent fractionation of layer 2/3→5B excitatory signaling in mouse auditory cortex
Abstract
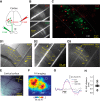
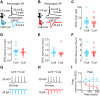
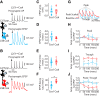
Auditory cortex (AC) layer 5B (L5B) contains both corticocollicular neurons, a type of pyramidal-tract neuron projecting to the inferior colliculus, and corticocallosal neurons, a type of intratelencephalic neuron projecting to contralateral AC. Although it is known that these neuronal types have distinct roles in auditory processing and different response properties to sound, the synaptic and intrinsic mechanisms shaping their input-output functions remain less understood. Here, we recorded in brain slices of mouse AC from retrogradely labeled corticocollicular and neighboring corticocallosal neurons in L5B. Corticocollicular neurons had, on average, lower input resistance, greater hyperpolarization-activated current (Ih), depolarized resting membrane potential, faster action potentials, initial spike doublets, and less spike-frequency adaptation. In paired recordings between single L2/3 and labeled L5B neurons, the probabilities of connection, amplitude, latency, rise time, and decay time constant of the unitary EPSC were not different for L2/3→corticocollicular and L2/3→corticocallosal connections. However, short trains of unitary EPSCs showed no synaptic depression in L2/3→corticocollicular connections, but substantial depression in L2/3→corticocallosal connections. Synaptic potentials in L2/3→corticocollicular connections decayed faster and showed less temporal summation, consistent with increased Ih in corticocollicular neurons, whereas synaptic potentials in L2/3→corticocallosal connections showed more temporal summation. Extracellular L2/3 stimulation at two different rates resulted in spiking in L5B neurons; for corticocallosal neurons the spike rate was frequency dependent, but for corticocollicular neurons it was not. Together, these findings identify cell-specific intrinsic and synaptic mechanisms that divide intracortical synaptic excitation from L2/3 to L5B into two functionally distinct pathways with different input-output functions.
Keywords: auditory cortex; cortical mechanisms; intrinsic mechanisms; short-term plasticity; synaptic mechanisms.
Copyright © 2015 the authors 0270-6474/15/353112-12$15.00/0.
Figures




Similar articles
-
Cell-Specific Cholinergic Modulation of Excitability of Layer 5B Principal Neurons in Mouse Auditory Cortex.J Neurosci. 2016 Aug 10;36(32):8487-99. doi: 10.1523/JNEUROSCI.0780-16.2016. J Neurosci. 2016. PMID: 27511019 Free PMC article.
-
Thalamocortical and Intracortical Inputs Differentiate Layer-Specific Mouse Auditory Corticocollicular Neurons.J Neurosci. 2019 Jan 9;39(2):256-270. doi: 10.1523/JNEUROSCI.3352-17.2018. Epub 2018 Oct 25. J Neurosci. 2019. PMID: 30361396 Free PMC article.
-
Novel GABAergic circuits mediating excitation/inhibition of Cajal-Retzius cells in the developing hippocampus.J Neurosci. 2013 Mar 27;33(13):5486-98. doi: 10.1523/JNEUROSCI.5680-12.2013. J Neurosci. 2013. PMID: 23536064 Free PMC article.
-
The auditory corticocollicular system: molecular and circuit-level considerations.Hear Res. 2014 Aug;314:51-9. doi: 10.1016/j.heares.2014.05.004. Epub 2014 Jun 7. Hear Res. 2014. PMID: 24911237 Free PMC article. Review.
-
Plasticity in the auditory system.Hear Res. 2018 May;362:61-73. doi: 10.1016/j.heares.2017.10.011. Epub 2017 Oct 31. Hear Res. 2018. PMID: 29126650 Review.
Cited by
-
Synaptic zinc potentiates AMPA receptor function in mouse auditory cortex.Cell Rep. 2023 Aug 29;42(8):112932. doi: 10.1016/j.celrep.2023.112932. Epub 2023 Aug 15. Cell Rep. 2023. PMID: 37585291 Free PMC article.
-
Traumatic Stress-Induced Increases in Anxiety-like Behavior and Alcohol Self-Administration Are Mediated by Central Amygdala CRF1 Neurons That Project to the Lateral Hypothalamus.J Neurosci. 2023 Dec 13;43(50):8690-8699. doi: 10.1523/JNEUROSCI.1414-23.2023. J Neurosci. 2023. PMID: 37932105 Free PMC article.
-
Auditory Corticofugal Neurons Transmit Auditory and Non-auditory Information During Behavior.J Neurosci. 2024 Feb 14;44(7):e1190232023. doi: 10.1523/JNEUROSCI.1190-23.2023. J Neurosci. 2024. PMID: 38123993 Free PMC article.
-
Cell-specific gain modulation by synaptically released zinc in cortical circuits of audition.Elife. 2017 Sep 9;6:e29893. doi: 10.7554/eLife.29893. Elife. 2017. PMID: 28887876 Free PMC article.
-
Rostral and caudal basolateral amygdala engage distinct circuits in the prelimbic and infralimbic prefrontal cortex.Elife. 2022 Dec 7;11:e82688. doi: 10.7554/eLife.82688. Elife. 2022. PMID: 36476757 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- F32 DC013734/DC/NIDCD NIH HHS/United States
- R01 NS061963/NS/NINDS NIH HHS/United States
- NS061963/NS/NINDS NIH HHS/United States
- R01 DC007905/DC/NIDCD NIH HHS/United States
- DC013272/DC/NIDCD NIH HHS/United States
- T32DC011499/DC/NIDCD NIH HHS/United States
- F32DC013734/DC/NIDCD NIH HHS/United States
- R03 DC012585/DC/NIDCD NIH HHS/United States
- T32 DC011499/DC/NIDCD NIH HHS/United States
- R03DC012585/DC/NIDCD NIH HHS/United States
- R56 DC013272/DC/NIDCD NIH HHS/United States
- DC007905/DC/NIDCD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
