Neural heterogeneities determine response characteristics to second-, but not first-order stimulus features
- PMID: 25698748
- PMCID: PMC4529327
- DOI: 10.1523/JNEUROSCI.3946-14.2015
Neural heterogeneities determine response characteristics to second-, but not first-order stimulus features
Abstract
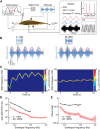
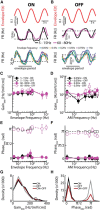
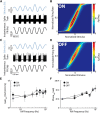
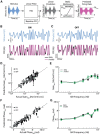
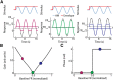
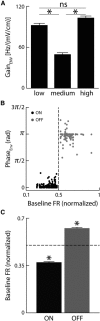
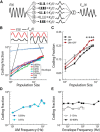
Neural heterogeneities are seen ubiquitously, but how they determine neural response properties remains unclear. Here we show that heterogeneities can either strongly, or not at all, influence neural responses to a given stimulus feature. Specifically, we recorded from peripheral electroreceptor neurons, which display strong heterogeneities in their resting discharge activity, in response to naturalistic stimuli consisting of a fast time-varying waveform (i.e., first-order) whose amplitude (i.e., second-order or envelope) varied slowly in the weakly electric fish Apteronotus leptorhynchus. Although electroreceptors displayed relatively homogeneous responses to first-order stimulus features, further analysis revealed two subpopulations with similar sensitivities that were excited or inhibited by increases in the envelope, respectively, for stimuli whose frequency content spanned the natural range. We further found that a linear-nonlinear cascade model incorporating the known linear response characteristics to first-order features and a static nonlinearity accurately reproduced experimentally observed responses to both first- and second-order features for all stimuli tested. Importantly, this model correctly predicted that the response magnitude is independent of either the stimulus waveform's or the envelope's frequency content. Further analysis of our model led to the surprising prediction that the mean discharge activity can be used to determine whether a given neuron is excited or inhibited by increases in the envelope. This prediction was validated by our experimental data. Thus, our results provide key insight as to how neural heterogeneities can determine response characteristics to some, but not other, behaviorally relevant stimulus features.
Keywords: electroreceptor; envelope; neural heterogeneity; weakly electric fish.
Copyright © 2015 the authors 0270-6474/15/353124-15$15.00/0.
Figures

Similar articles
-
Optimized Parallel Coding of Second-Order Stimulus Features by Heterogeneous Neural Populations.J Neurosci. 2016 Sep 21;36(38):9859-72. doi: 10.1523/JNEUROSCI.1433-16.2016. J Neurosci. 2016. PMID: 27656024 Free PMC article.
-
Neural heterogeneities influence envelope and temporal coding at the sensory periphery.Neuroscience. 2011 Jan 13;172:270-84. doi: 10.1016/j.neuroscience.2010.10.061. Epub 2010 Oct 28. Neuroscience. 2011. PMID: 21035523 Free PMC article.
-
Sparse and dense coding of natural stimuli by distinct midbrain neuron subpopulations in weakly electric fish.J Neurophysiol. 2011 Dec;106(6):3102-18. doi: 10.1152/jn.00588.2011. Epub 2011 Sep 21. J Neurophysiol. 2011. PMID: 21940609 Free PMC article.
-
Perception and coding of envelopes in weakly electric fishes.J Exp Biol. 2013 Jul 1;216(Pt 13):2393-402. doi: 10.1242/jeb.082321. J Exp Biol. 2013. PMID: 23761464 Free PMC article. Review.
-
SK channel subtypes enable parallel optimized coding of behaviorally relevant stimulus attributes: A review.Channels (Austin). 2017 Jul 4;11(4):281-304. doi: 10.1080/19336950.2017.1299835. Epub 2017 Mar 1. Channels (Austin). 2017. PMID: 28277938 Free PMC article. Review.
Cited by
-
Descending pathways mediate adaptive optimized coding of natural stimuli in weakly electric fish.Sci Adv. 2019 Oct 30;5(10):eaax2211. doi: 10.1126/sciadv.aax2211. eCollection 2019 Oct. Sci Adv. 2019. PMID: 31693006 Free PMC article.
-
Weak signal amplification and detection by higher-order sensory neurons.J Neurophysiol. 2016 Apr;115(4):2158-75. doi: 10.1152/jn.00811.2015. Epub 2016 Feb 3. J Neurophysiol. 2016. PMID: 26843601 Free PMC article.
-
Electrosensory processing in Apteronotus albifrons: implications for general and specific neural coding strategies across wave-type weakly electric fish species.J Neurophysiol. 2016 Dec 1;116(6):2909-2921. doi: 10.1152/jn.00594.2016. Epub 2016 Sep 28. J Neurophysiol. 2016. PMID: 27683890 Free PMC article.
-
Stimulus background influences phase invariant coding by correlated neural activity.Elife. 2017 Mar 18;6:e24482. doi: 10.7554/eLife.24482. Elife. 2017. PMID: 28315519 Free PMC article.
-
Recurrence-mediated suprathreshold stochastic resonance.J Comput Neurosci. 2021 Nov;49(4):407-418. doi: 10.1007/s10827-021-00788-3. Epub 2021 May 18. J Comput Neurosci. 2021. PMID: 34003421 Free PMC article.
References
-
- Bastian J. Electrolocation II: the effects of moving objects and other electrical stimuli on the activities of two categories of posterior lateral line lobe cells in Apteronotus albifrons. J Comp Physiol A. 1981;144:481–494. doi: 10.1007/BF01326833. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
