Overexpression of Sox11 promotes corticospinal tract regeneration after spinal injury while interfering with functional recovery
- PMID: 25698749
- PMCID: PMC4331631
- DOI: 10.1523/JNEUROSCI.2832-14.2015
Overexpression of Sox11 promotes corticospinal tract regeneration after spinal injury while interfering with functional recovery
Abstract
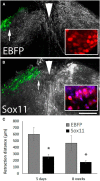
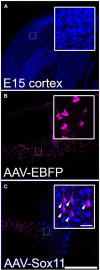
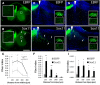
Embryonic neurons, peripheral neurons, and CNS neurons in zebrafish respond to axon injury by initiating pro-regenerative transcriptional programs that enable axons to extend, locate appropriate targets, and ultimately contribute to behavioral recovery. In contrast, many long-distance projection neurons in the adult mammalian CNS, notably corticospinal tract (CST) neurons, display a much lower regenerative capacity. To promote CNS repair, a long-standing goal has been to activate pro-regenerative mechanisms that are normally missing from injured CNS neurons. Sox11 is a transcription factor whose expression is common to a many types of regenerating neurons, but it is unknown whether suboptimal Sox11 expression contributes to low regenerative capacity in the adult mammalian CNS. Here we show in adult mice that dorsal root ganglion neurons (DRGs) and CST neurons fail to upregulate Sox11 after spinal axon injury. Furthermore, forced viral expression of Sox11 reduces axonal dieback of DRG axons, and promotes CST sprouting and regenerative axon growth in both acute and chronic injury paradigms. In tests of forelimb dexterity, however, Sox11 overexpression in the cortex caused a modest but consistent behavioral impairment. These data identify Sox11 as a key transcription factor that can confer an elevated innate regenerative capacity to CNS neurons. The results also demonstrate an unexpected dissociation between axon growth and behavioral outcome, highlighting the need for additional strategies to optimize the functional output of stimulated neurons.
Keywords: Sox11; axon regeneration; gene therapy; spinal cord injury; transcription factor.
Copyright © 2015 the authors 0270-6474/15/353139-07$15.00/0.
Conflict of interest statement
The authors declare no conflicting financial interests.
Figures

Similar articles
-
Optogenetic Interrogation of Functional Synapse Formation by Corticospinal Tract Axons in the Injured Spinal Cord.J Neurosci. 2016 May 25;36(21):5877-90. doi: 10.1523/JNEUROSCI.4203-15.2016. J Neurosci. 2016. PMID: 27225775 Free PMC article.
-
Pten Deletion Promotes Regrowth of Corticospinal Tract Axons 1 Year after Spinal Cord Injury.J Neurosci. 2015 Jul 1;35(26):9754-63. doi: 10.1523/JNEUROSCI.3637-14.2015. J Neurosci. 2015. PMID: 26134657 Free PMC article.
-
Sprouting of axonal collaterals after spinal cord injury is prevented by delayed axonal degeneration.Exp Neurol. 2014 Nov;261:451-61. doi: 10.1016/j.expneurol.2014.07.014. Epub 2014 Jul 28. Exp Neurol. 2014. PMID: 25079366
-
The Dorsal Column Lesion Model of Spinal Cord Injury and Its Use in Deciphering the Neuron-Intrinsic Injury Response.Dev Neurobiol. 2018 Oct;78(10):926-951. doi: 10.1002/dneu.22601. Epub 2018 May 11. Dev Neurobiol. 2018. PMID: 29717546 Free PMC article. Review.
-
Intrinsic regulation of axon regeneration after spinal cord injury: Recent advances and remaining challenges.Exp Neurol. 2022 Nov;357:114198. doi: 10.1016/j.expneurol.2022.114198. Epub 2022 Aug 6. Exp Neurol. 2022. PMID: 35944658 Review.
Cited by
-
The Role of Lipids, Lipid Metabolism and Ectopic Lipid Accumulation in Axon Growth, Regeneration and Repair after CNS Injury and Disease.Cells. 2021 May 1;10(5):1078. doi: 10.3390/cells10051078. Cells. 2021. PMID: 34062747 Free PMC article. Review.
-
Regulation of axonal regeneration after mammalian spinal cord injury.Nat Rev Mol Cell Biol. 2023 Jun;24(6):396-413. doi: 10.1038/s41580-022-00562-y. Epub 2023 Jan 5. Nat Rev Mol Cell Biol. 2023. PMID: 36604586 Review.
-
Increased Expression of Transcription Factor SRY-box-Containing Gene 11 (Sox11) Enhances Neurite Growth by Regulating Neurotrophic Factor Responsiveness.Neuroscience. 2018 Jul 1;382:93-104. doi: 10.1016/j.neuroscience.2018.04.037. Epub 2018 May 8. Neuroscience. 2018. PMID: 29746989 Free PMC article.
-
Recovery of corticospinal tract injured by traumatic axonal injury at the subcortical white matter: a case report.Neural Regen Res. 2016 Sep;11(9):1527-1528. doi: 10.4103/1673-5374.191230. Neural Regen Res. 2016. PMID: 27857761 Free PMC article. No abstract available.
-
Ascending dorsal column sensory neurons respond to spinal cord injury and downregulate genes related to lipid metabolism.Sci Rep. 2021 Jan 11;11(1):374. doi: 10.1038/s41598-020-79624-0. Sci Rep. 2021. PMID: 33431991 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
