Spiral ganglion degeneration and hearing loss as a consequence of satellite cell death in saposin B-deficient mice
- PMID: 25698761
- PMCID: PMC6605599
- DOI: 10.1523/JNEUROSCI.3920-13.2015
Spiral ganglion degeneration and hearing loss as a consequence of satellite cell death in saposin B-deficient mice
Abstract
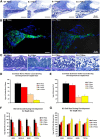
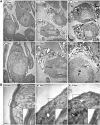
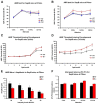
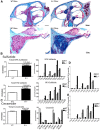
Saposin B (Sap B) is an essential activator protein for arylsulfatase A in the hydrolysis of sulfatide, a lipid component of myelin. To study Sap B's role in hearing and balance, a Sap B-deficient (B(-/-)) mouse was evaluated. At both light and electron microscopy (EM) levels, inclusion body accumulation was seen in satellite cells surrounding spiral ganglion (SG) neurons from postnatal month 1 onward, progressing into large vacuoles preceding satellite cell degeneration, and followed by SG degeneration. EM also revealed reduced or absent myelin sheaths in SG neurons from postnatal month 8 onwards. Hearing loss was initially seen at postnatal month 6 and progressed thereafter for frequency-specific stimuli, whereas click responses became abnormal from postnatal month 13 onward. The progressive hearing loss correlated with the accumulation of inclusion bodies in the satellite cells and their subsequent degeneration. Outer hair cell numbers and efferent function measures (distortion product otoacoustic emissions and contralateral suppression) were normal in the B(-/-) mice throughout this period. Alcian blue staining of SGs demonstrated that these inclusion bodies corresponded to sulfatide accumulation. In contrast, changes in the vestibular system were much milder, but caused severe physiologic deficits. These results demonstrate that loss of Sap B function leads to progressive sulfatide accumulation in satellite cells surrounding the SG neurons, leading to satellite cell degeneration and subsequent SG degeneration with a resultant loss of hearing. Relative sparing of the efferent auditory and vestibular neurons suggests that alternate glycosphingolipid metabolic pathways predominate in these other systems.
Keywords: auditory; cochlea; prosaposin; saposin B; spiral ganglion; sulfatide.
Copyright © 2015 the authors 0270-6474/15/353263-13$15.00/0.
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
