Galangin, a novel dietary flavonoid, attenuates metastatic feature via PKC/ERK signaling pathway in TPA-treated liver cancer HepG2 cells
- PMID: 25698902
- PMCID: PMC4332891
- DOI: 10.1186/s12935-015-0168-2
Galangin, a novel dietary flavonoid, attenuates metastatic feature via PKC/ERK signaling pathway in TPA-treated liver cancer HepG2 cells
Abstract
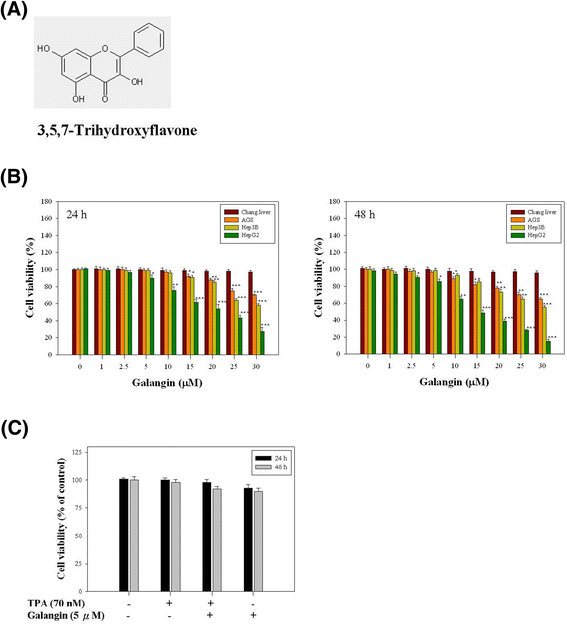
Background: Galangin (3,5,7-trihydroxyflavone) is a flavonoid compound found in high concentration in lesser galangal. The objective of this study was to investigate the ability of galangin to inhibit 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced the invasion and metastasis of HepG2 liver cancer cells.
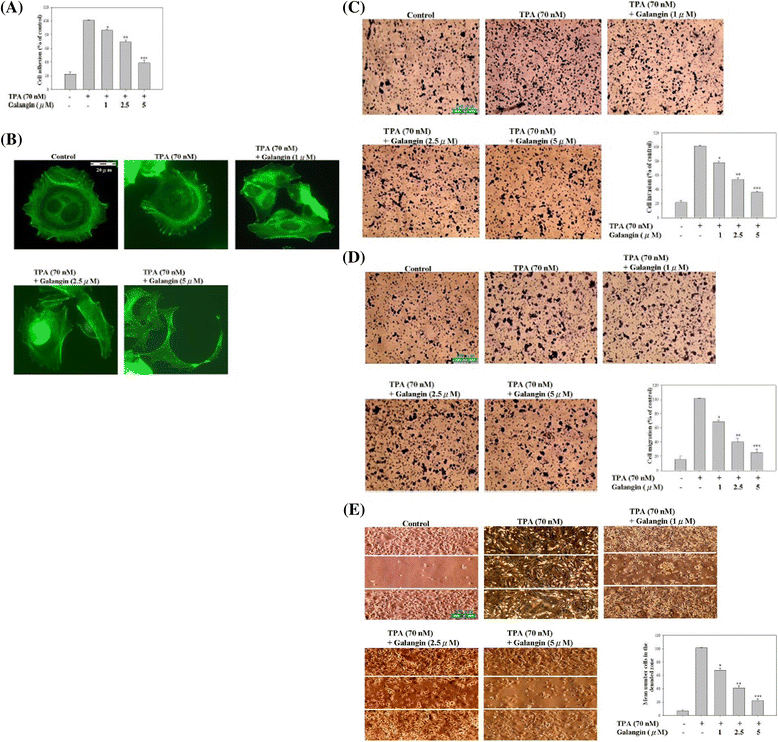
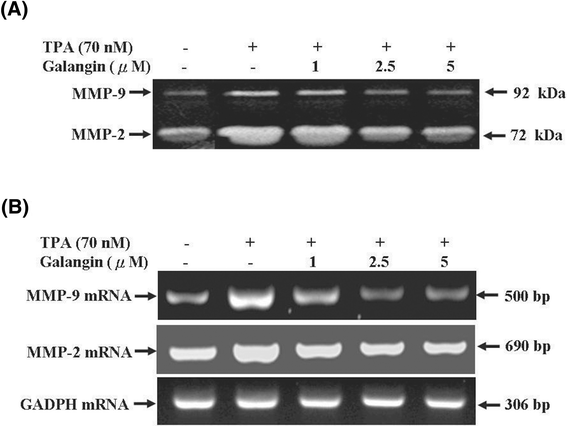
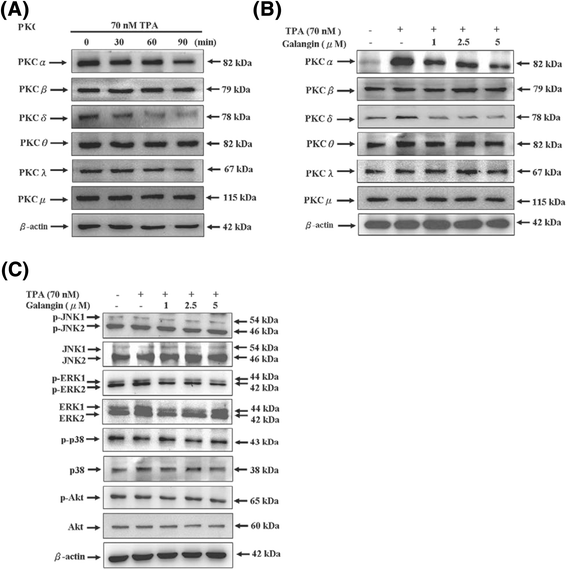
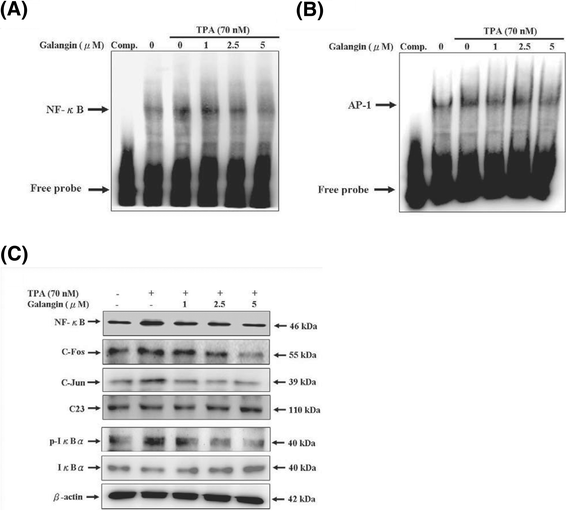
Results: First, using a cell-matrix adhesion assay, immunofluorescence assay, transwell-chamber invasion/migration assay, and wound healing assay, we observed that galangin exerted an inhibitory effect on TPA-induced cell adhesion, morphology/actin cytoskeleton arrangement, invasion and migration. Furthermore, the results of gelatin zymography and reverse transcriptase polymerase chain reaction (RT-PCR) assays showed that galangin reduced the TPA-induced enzyme activity of matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9) in HepG2 cells; moreover, the messenger RNA level was downregulated. We also observed through a Western blotting assay that galangin strongly inhibited the TPA-induced protein expressions of protein kinase Cα (PKCα), protein kinase Cδ (PKCδ), phosphorylated extracellular signal-regulated kinase 1/2 (ERK1/2), the phospho-inhibitor of kappaBα (phospho-IκBα), c-Fos, c-Jun, and nuclear factor kappa B (NF-κB). Next, galangin dose-dependently inhibited the binding ability of NF-κB and activator protein 1 (AP-1) to MMP-2/MMP-9 promoters, respectively, resulting in the suppression of MMP-2/MMP-9 enzyme activity.
Conclusions: The results revealed that galangin effectively inhibited the TPA-induced invasion and migration of HepG2 cells through a protein kinase C/extracellular signal-regulated kinase (PKC/ERK) pathway. Thus, galangin may have widespread applications in clinical therapy as an anti-metastatic medicament.
Keywords: ERK; Galangin; Invasion; MMP-2; MMP-9; Migration; PKC-α; TPA.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

