Cellular cytokine and chemokine responses to parasite antigens and fungus and mite allergens in children co-infected with helminthes and protozoa parasites
- PMID: 25698903
- PMCID: PMC4334608
- DOI: 10.1186/s12950-015-0050-y
Cellular cytokine and chemokine responses to parasite antigens and fungus and mite allergens in children co-infected with helminthes and protozoa parasites
Abstract
Background: In sub-Saharan Africa poly-parasite infections are frequently observed in children, and with poly-parasitism modulating immune mechanisms, mediated by cytokines and chemokines, are required to prevent overwhelming inflammation and host tissue damage. We analyzed in children co-infected with helminthes and protozoan parasites their cellular production of regulatory and pro-inflammatory cytokines and chemokines in response to parasite antigens and allergens.
Methods: Intestinal and intravascular parasite infections were detected in stool and urines samples. The in vitro cellular cytokine and chemokine responses of peripheral blood mononuclear cells (PBMC) to parasite antigens and allergens were analysed in children (n = 87) with single and poly-parasite infection, and skin prick test reactivity to fungus and mite allergens was determined in singly and poly-parasitized children (n = 509).
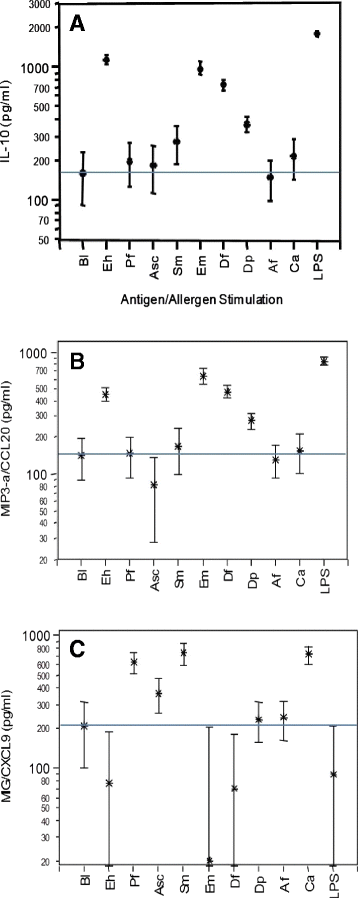
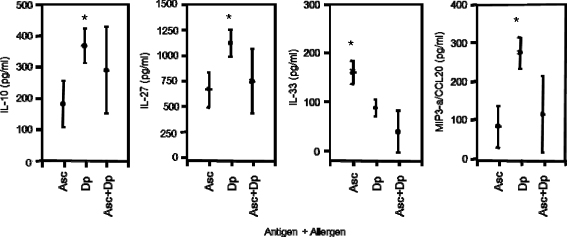
Results: In children Entamoeba histolytica/dispar (62%), Necator americanus (31%), Schistosoma haematobium (28%), S. mansoni (21%), Hymenolepis nana (2%) and Strongyloides stercoralis (1%) were diagnosed. Singly infected were 37%, 47% were positive for 2 or more parasite species and 16% were infection-free. When PBMC were stimulated in vitro with parasite antigens and allergens, regulatory-type cytokine IL-27 and alarmin-type IL-33 enhanced with poly-parasite infections whilst IL-10 and pro-inflammatory MIP3-α/CCL20 and MIG/CXCL9 were produced in similar amounts in singly or poly-parasitized children. The co-stimulation in vitro of PBMC with mite allergens and Ascaris lumbricoides antigens depressed the allergen-induced pro-inflammatory IL-27, IL-33 and MIP3-α/CCL20 responses while regulatory IL-10 remained unaffected. Post albendazole and/or praziquantel treatment, the cellular release of IL-10, IL-33, MIP3-α/CCL20 and MIG/CXCL9 lessened significantly in all children infection groups. Skin prick test (SPT) reactivity to fungus Aspergillus fumigatus and mite Dermatophagoides pteronyssinus allergens was investigated in 509 children, and positive SPT responses were found in 23% of the infection-free, and in 47%, 53% and 56% of the singly, doubly and poly-parasite infected, respectively.
Conclusions: In children co-infected with helminthes and protozoan parasites a mixed cellular response profile of both inflammatory and regulatory chemokines and cytokines was stimulated by individual antigens and allergens, pro-inflammatory cytokines and chemokines enhanced with an increasing number of parasite infections, and in poly-parasitized children skin prick test reactivity to allergens extracts was highest.
Keywords: Allergen; Amoebiasis; Chemokine; Co-infection; Cytokine; Hookworm; Schistosomiasis; Skin prick test.
Figures



Similar articles
-
Cytokine and chemokine responses to helminth and protozoan parasites and to fungus and mite allergens in neonates, children, adults, and the elderly.Immun Ageing. 2013 Jul 15;10(1):29. doi: 10.1186/1742-4933-10-29. Immun Ageing. 2013. PMID: 23855879 Free PMC article.
-
Cytokine and chemokine responses in patients co-infected with Entamoeba histolytica/dispar, Necator americanus and Mansonella perstans and changes after anti-parasite treatment.Microbes Infect. 2006 Jan;8(1):238-47. doi: 10.1016/j.micinf.2005.06.019. Epub 2005 Sep 12. Microbes Infect. 2006. PMID: 16239120
-
Coinfections with Schistosoma haematobium, Necator americanus, and Entamoeba histolytica/Entamoeba dispar in children: chemokine and cytokine responses and changes after antiparasite treatment.J Infect Dis. 2009 Jun 1;199(11):1583-91. doi: 10.1086/598950. J Infect Dis. 2009. PMID: 19392635
-
Parasite allergens.Mol Immunol. 2018 Aug;100:113-119. doi: 10.1016/j.molimm.2018.03.014. Epub 2018 Mar 24. Mol Immunol. 2018. PMID: 29588070 Review.
-
Human schistosomiasis decreases immune responses to allergens and clinical manifestations of asthma.Chem Immunol Allergy. 2006;90:29-44. doi: 10.1159/000088879. Chem Immunol Allergy. 2006. PMID: 16210901 Review.
Cited by
-
Beyond schistosomiasis: unraveling co-infections and altered immunity.Clin Microbiol Rev. 2024 Mar 14;37(1):e0009823. doi: 10.1128/cmr.00098-23. Epub 2024 Feb 6. Clin Microbiol Rev. 2024. PMID: 38319102 Free PMC article. Review.
-
The Impact of HIV and Parasite Single Infection and Coinfection on Telomere Length: A Systematic Review.Curr Issues Mol Biol. 2024 Jul 8;46(7):7258-7290. doi: 10.3390/cimb46070431. Curr Issues Mol Biol. 2024. PMID: 39057072 Free PMC article. Review.
-
Age affects antibody levels and anthelmintic treatment efficacy in a wild rodent.Int J Parasitol Parasites Wildl. 2019 Mar 14;8:240-247. doi: 10.1016/j.ijppaw.2019.03.004. eCollection 2019 Apr. Int J Parasitol Parasites Wildl. 2019. PMID: 30923672 Free PMC article.
-
Plasmodium and intestinal parasite perturbations of the infected host's inflammatory responses: a systematic review.Parasit Vectors. 2018 Jul 3;11(1):387. doi: 10.1186/s13071-018-2948-8. Parasit Vectors. 2018. PMID: 29970128 Free PMC article.
-
Immunization with Hydatid Cyst Wall Antigens Can Inhibit Breast Cancer through Changes in Serum Levels of Th1/Th2 Cytokines.Int J Prev Med. 2020 Dec 11;11:189. doi: 10.4103/ijpvm.IJPVM_311_19. eCollection 2020. Int J Prev Med. 2020. PMID: 33815713 Free PMC article.
References
-
- Buck AA, Anderson RI, MacRae AA. Epidemiology of poly-parasitism. II. Types of combinations, relative frequency and associations of multiple infections. Tropenmed Parasitol. 1978;29:137–44. - PubMed
-
- Hamm DM, Agossou A, Gantin RG, Kocherscheidt L, Banla M, Dietz K, Soboslay PT. Coinfections with Schistosoma haematobium, Necator americanus, and Entamoeba histolytica/Entamoeba dispar in children: chemokine and cytokine responses and changes after antiparasite treatment. J Infect Dis. 2009;199:1583–91. doi: 10.1086/598950. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

