Model of Cation Transportation Mediated by High-Affinity Potassium Transporters (HKTs) in Higher Plants
- PMID: 25698907
- PMCID: PMC4334588
- DOI: 10.1186/s12575-014-0013-3
Model of Cation Transportation Mediated by High-Affinity Potassium Transporters (HKTs) in Higher Plants
Erratum in
-
Erratum to: Model of Cation Transportation Mediated by High-Affinity Potassium Transporters (HKTs) in Higher Plants.Biol Proced Online. 2015 Apr 30;17:9. doi: 10.1186/s12575-015-0021-y. eCollection 2015. Biol Proced Online. 2015. PMID: 25931989 Free PMC article.
Abstract
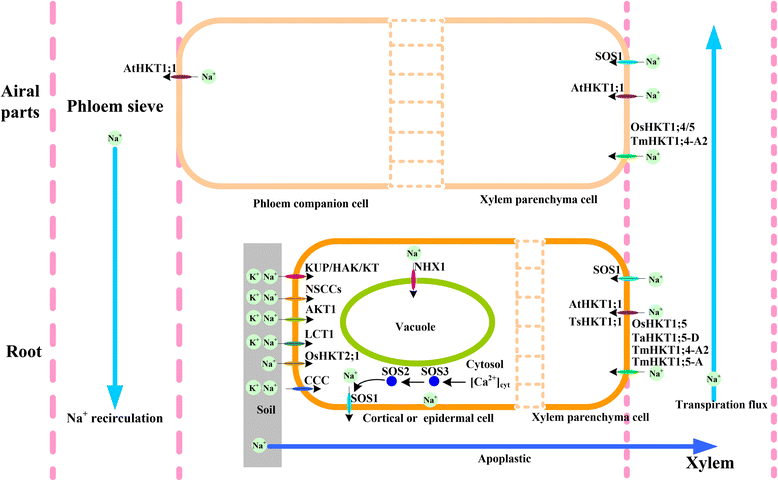
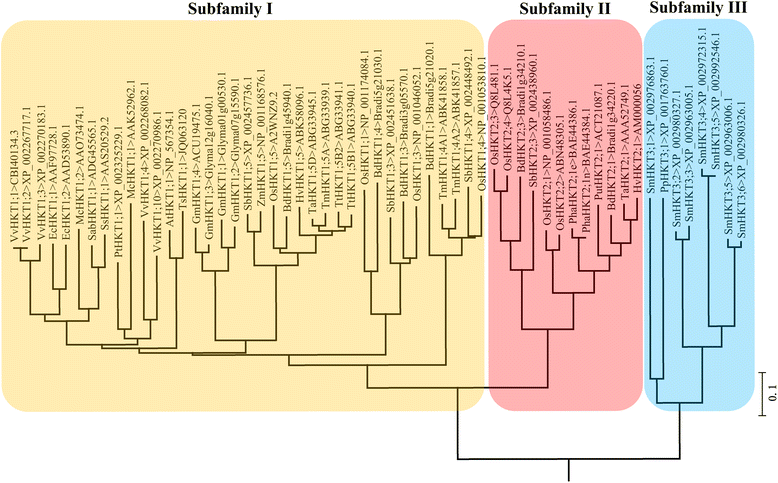
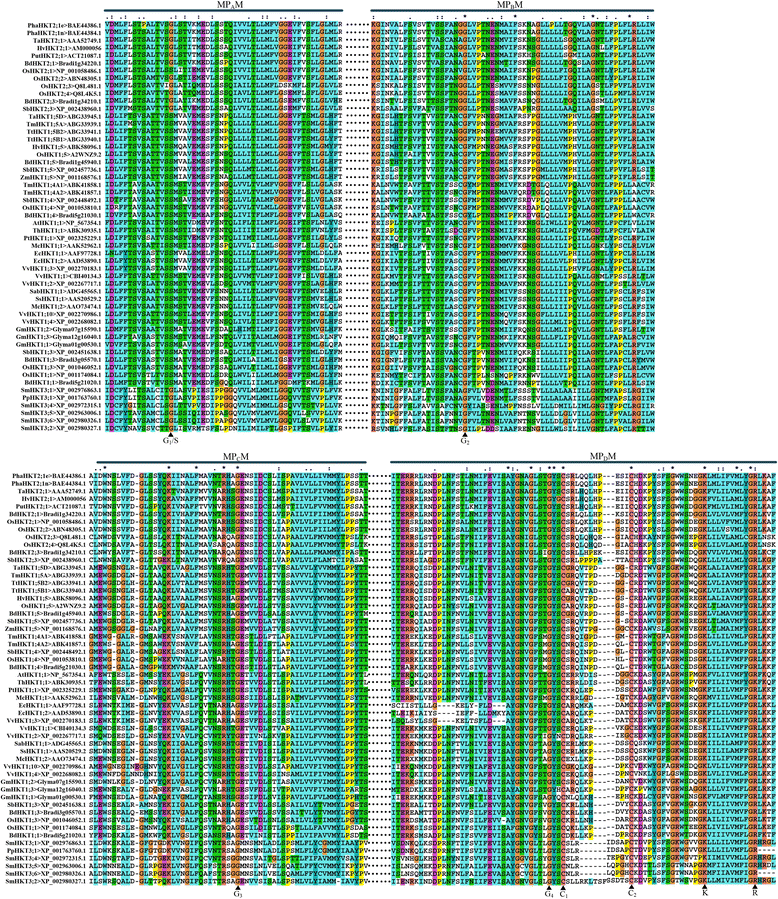
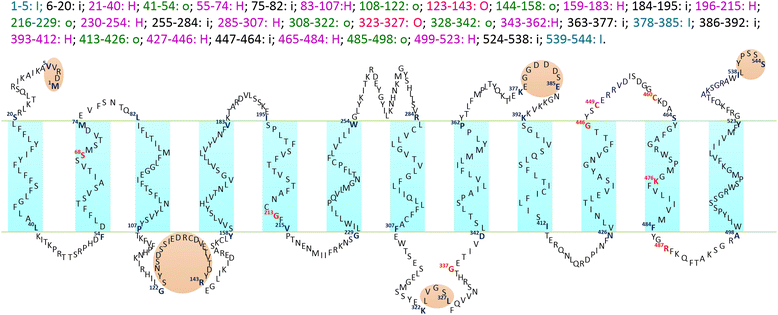
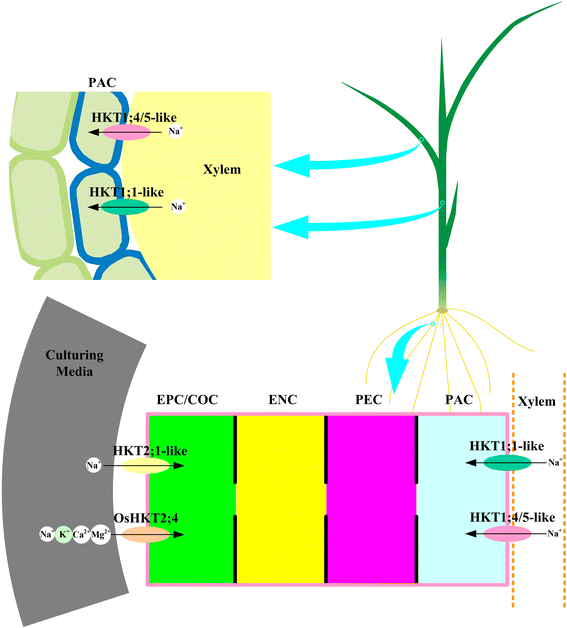
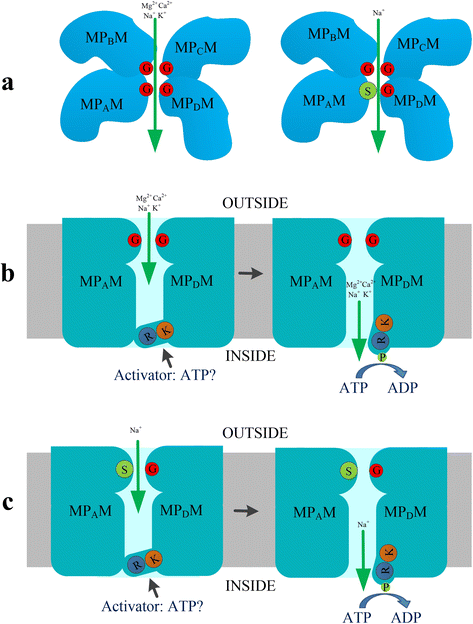
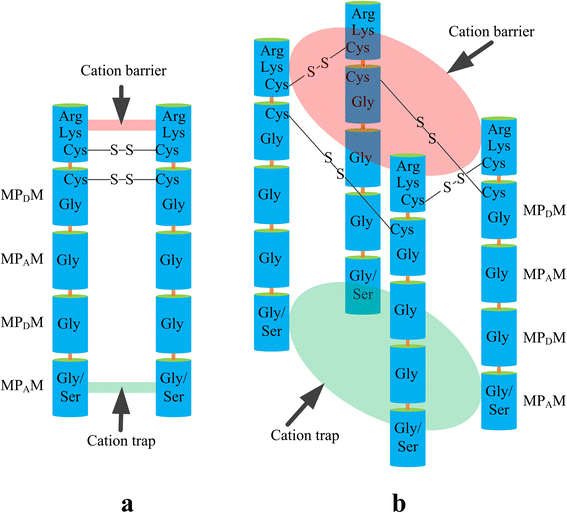
Trk/Ktr/HKT transporters probably were evolved from simple K(+) channels KcsA. HKT transporters, which mediate Na(+)-uniport or Na(+)/K(+)-symport, maintain K(+)/Na(+) homeostasis and increase salinity tolerance, can be classified into three subfamilies in higher plants. In this review, we systematically analyzed the characteristics of amino acids sequences and physiological functions of HKT transporters in higher plant. Furthermore, we depicted the hypothetical models of cations selection and transportation mediated by HKT transporters according to the highly conserved structure for the goal of better understanding the cations transportation processes.
Keywords: Cation transport; HKT transporters; K+/Na+ homeostasis; Na+-uniport; Na+/K+-symport.
Figures
References
-
- Sobbarao GV, Ito O, Berry WL, Wheeler RM. Sodium-A functional plant nutrient. Crit Rev Plant Sci. 2003;22:391–416.
-
- Kronzucker HJ, Coskun D, Schulze LM, Wong JR, Britto DT. Sodium as nutrient and toxicant. Plant Soil. 2013;369:1–23.
-
- Maathuis FJ. Sodium in plants: perception, signalling, and regulation of sodium fluxes. J Exp Bot. 2014;65:849–58. - PubMed
-
- Maathuis FJM, Sanders D. Energization of potassium uptake in Arabidopsis thaliana. Planta. 1993;191:302–7.
-
- Rodríguez-Navarro A. Potassium transport in fungi and plants. BBA-Proteins Proteom. 2000;1469:1–30. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

