Optical triggered seizures using a caged 4-Aminopyridine
- PMID: 25698919
- PMCID: PMC4316705
- DOI: 10.3389/fnins.2015.00025
Optical triggered seizures using a caged 4-Aminopyridine
Abstract
Animal models of epilepsy are critical not only for understanding the fundamental mechanism of epilepsy but also for testing the efficacy of new antiepileptic drugs and novel therapeutic interventions. Photorelease of caged molecules is widely used in biological research to control pharmacologic events with high spatio-temporal resolution. We developed a technique for in vivo optical triggering of neocortical seizures using a novel caged compound based on ruthenium photochemistry (RuBi-4AP). Epileptiform events in mouse cortex were induced with blue light in both whole brain and focal illumination. Multi-electrode array recording and optical techniques were used to characterize the propagation of these epileptic events, including interictal spikes, polyspikes, and ictal discharges. These results demonstrate a novel optically-triggered seizure model, with high spatio-temporal control, that could have widespread application in the investigation of ictal onset, propagation and to develop novel light-based therapeutic interventions.
Keywords: caged compound; electrophysiology; epilepsy model; neocortex; optical imaging; photostimulation.
Figures




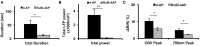
Similar articles
-
Optical control of focal epilepsy in vivo with caged γ-aminobutyric acid.Ann Neurol. 2012 Jan;71(1):68-75. doi: 10.1002/ana.22596. Ann Neurol. 2012. PMID: 22275253 Free PMC article.
-
Centre of epileptogenic tubers generate and propagate seizures in tuberous sclerosis.Brain. 2016 Oct;139(Pt 10):2653-2667. doi: 10.1093/brain/aww192. Epub 2016 Aug 6. Brain. 2016. PMID: 27497492
-
Photolysis of Caged-GABA Rapidly Terminates Seizures In Vivo: Concentration and Light Intensity Dependence.Front Neurol. 2017 May 18;8:215. doi: 10.3389/fneur.2017.00215. eCollection 2017. Front Neurol. 2017. PMID: 28572790 Free PMC article.
-
Do interictal discharges promote or control seizures? Experimental evidence from an in vitro model of epileptiform discharge.Epilepsia. 2001;42 Suppl 3:2-4. doi: 10.1046/j.1528-1157.2001.042suppl.3002.x. Epilepsia. 2001. PMID: 11520313 Review.
-
Interictal-ictal interactions and limbic seizure generation.Rev Neurol (Paris). 1999 Jul;155(6-7):468-71. Rev Neurol (Paris). 1999. PMID: 10472661 Review.
Cited by
-
Theoretical Design, Synthesis, and In Vitro Neurobiological Applications of a Highly Efficient Two-Photon Caged GABA Validated on an Epileptic Case.ACS Omega. 2021 Jun 3;6(23):15029-15045. doi: 10.1021/acsomega.1c01164. eCollection 2021 Jun 15. ACS Omega. 2021. PMID: 34151084 Free PMC article.
-
Photopharmacological modulation of hippocampal local field potential by caged-glutamate with MicroLED probe.Neuropsychopharmacol Rep. 2024 Sep;44(3):658-662. doi: 10.1002/npr2.12472. Epub 2024 Aug 9. Neuropsychopharmacol Rep. 2024. PMID: 39126158 Free PMC article.
-
In Vivo Neuropharmacological Effects of Neophytadiene.Molecules. 2023 Apr 14;28(8):3457. doi: 10.3390/molecules28083457. Molecules. 2023. PMID: 37110691 Free PMC article.
-
Gabrb3 is required for the functional integration of pyramidal neuron subtypes in the somatosensory cortex.Neuron. 2023 Jan 18;111(2):256-274.e10. doi: 10.1016/j.neuron.2022.10.037. Epub 2022 Nov 28. Neuron. 2023. PMID: 36446382 Free PMC article.
-
Synthesis and application of a photocaged-L-lactate for studying the biological roles of L-lactate.Commun Chem. 2025 Apr 5;8(1):104. doi: 10.1038/s42004-025-01495-1. Commun Chem. 2025. PMID: 40188278 Free PMC article.
References
-
- Barkai E., Friedman A., Grossman Y., Gutnick M. J. (1995). Laminar pattern of synaptic inhibition during convulsive activity induced by 4-aminopyridine in neocortical slices. J. Neurophysiol. 73, 1462–1467. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

