Highly efficient method for gene delivery into mouse dorsal root ganglia neurons
- PMID: 25698920
- PMCID: PMC4313362
- DOI: 10.3389/fnmol.2015.00002
Highly efficient method for gene delivery into mouse dorsal root ganglia neurons
Erratum in
-
Erratum on: Highly efficient method for gene delivery into mouse dorsal root ganglia neurons.Front Mol Neurosci. 2015 May 18;8:16. doi: 10.3389/fnmol.2015.00016. eCollection 2015. Front Mol Neurosci. 2015. PMID: 26041989 Free PMC article.
Abstract
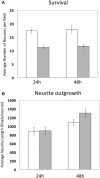
The development of gene transfection technologies has greatly advanced our understanding of life sciences. While use of viral vectors has clear efficacy, it requires specific expertise and biological containment conditions. Electroporation has become an effective and commonly used method for introducing DNA into neurons and in intact brain tissue. The present study describes the use of the Neon® electroporation system to transfect genes into dorsal root ganglia neurons isolated from embryonic mouse Day 13.5-16. This cell type has been particularly recalcitrant and refractory to physical or chemical methods for introduction of DNA. By optimizing the culture condition and parameters including voltage and duration for this specific electroporation system, high efficiency (60-80%) and low toxicity (>60% survival) were achieved with robust differentiation in response to Nerve growth factor (NGF). Moreover, 3-50 times fewer cells are needed (6 × 10(4)) compared with other traditional electroporation methods. This approach underlines the efficacy of this type of electroporation, particularly when only limited amount of cells can be obtained, and is expected to greatly facilitate the study of gene function in dorsal root ganglia neuron cultures.
Keywords: EGFP expression; Nerve growth factor (NGF); dorsal root ganglion (DRG) neuron; electroporation; gene expression; nucleofection; primary neurons; transfection.
Figures



References
-
- Butcher A. J., Torrecilla I., Young K. W., Kong K. C., Mistry S. C., Bottrill A. R., et al. (2009). N-methyl-D-aspartate receptors mediate the phosphorylation and desensitization of muscarinic receptors in cerebellar granule neurons. J. Biol. Chem. 284, 17147–17156. 10.1074/jbc.M901031200 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

