Expression of ionotropic receptors in terrestrial hermit crab's olfactory sensory neurons
- PMID: 25698921
- PMCID: PMC4313712
- DOI: 10.3389/fncel.2014.00448
Expression of ionotropic receptors in terrestrial hermit crab's olfactory sensory neurons
Abstract
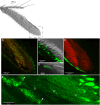
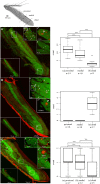
Coenobitidae are one out of at least five crustacean lineages which independently succeeded in the transition from water to land. This change in lifestyle required adaptation of the peripheral olfactory organs, the antennules, in order to sense chemical cues in the new terrestrial habitat. Hermit crab olfactory aesthetascs are arranged in a field on the distal segment of the antennular flagellum. Aesthetascs house approximately 300 dendrites with their cell bodies arranged in spindle-like complexes of ca. 150 cell bodies each. While the aesthetascs of aquatic crustaceans have been shown to be the place of odor uptake and previous studies identified ionotropic receptors (IRs) as the putative chemosensory receptors expressed in decapod antennules, the expression of IRs besides the IR co-receptors IR25a and IR93a in olfactory sensory neurons (OSNs) has not been documented yet. Our goal was to reveal the expression and distribution pattern of non-co-receptor IRs in OSNs of Coenobita clypeatus, a terrestrial hermit crab, with RNA in situ hybridization. We expanded our previously published RNAseq dataset, and revealed 22 novel IR candidates in the Coenobita antennules. We then used RNA probes directed against three different IRs to visualize their expression within the OSN cell body complexes. Furthermore we aimed to characterize ligand spectra of single aesthetascs by recording local field potentials and responses from individual dendrites. This also allowed comparison to functional data from insect OSNs expressing antennal IRs. We show that this orphan receptor subgroup with presumably non-olfactory function in insects is likely the basis of olfaction in terrestrial hermit crabs.
Keywords: antennules; crustacea; electrophysiology; in situ hybridization; ionotropic receptors; olfaction.
Figures




Similar articles
-
The hermit crab's nose-antennal transcriptomics.Front Neurosci. 2014 Jan 21;7:266. doi: 10.3389/fnins.2013.00266. eCollection 2013. Front Neurosci. 2014. PMID: 24478616 Free PMC article.
-
Central projections of antennular chemosensory and mechanosensory afferents in the brain of the terrestrial hermit crab (Coenobita clypeatus; Coenobitidae, Anomura).Front Neuroanat. 2015 Jul 15;9:94. doi: 10.3389/fnana.2015.00094. eCollection 2015. Front Neuroanat. 2015. PMID: 26236202 Free PMC article.
-
Single cell transcriptomes reveal expression patterns of chemoreceptor genes in olfactory sensory neurons of the Caribbean spiny lobster, Panulirus argus.BMC Genomics. 2020 Sep 22;21(1):649. doi: 10.1186/s12864-020-07034-7. BMC Genomics. 2020. PMID: 32962631 Free PMC article.
-
More than one way to smell ashore - Evolution of the olfactory pathway in terrestrial malacostracan crustaceans.Arthropod Struct Dev. 2021 Jan;60:101022. doi: 10.1016/j.asd.2020.101022. Epub 2020 Dec 29. Arthropod Struct Dev. 2021. PMID: 33385761 Review.
-
Crustacean olfactory systems: A comparative review and a crustacean perspective on olfaction in insects.Prog Neurobiol. 2018 Feb;161:23-60. doi: 10.1016/j.pneurobio.2017.11.005. Epub 2017 Dec 2. Prog Neurobiol. 2018. PMID: 29197652 Review.
Cited by
-
An enteric neuron-expressed variant ionotropic receptor detects ingested salts to regulate salt stress resistance.bioRxiv [Preprint]. 2025 May 8:2025.04.11.648259. doi: 10.1101/2025.04.11.648259. bioRxiv. 2025. PMID: 40391324 Free PMC article. Preprint.
-
Ionotropic Receptors Identified within the Tentacle of the Freshwater Snail Biomphalaria glabrata, an Intermediate Host of Schistosoma mansoni.PLoS One. 2016 Jun 2;11(6):e0156380. doi: 10.1371/journal.pone.0156380. eCollection 2016. PLoS One. 2016. PMID: 27253696 Free PMC article.
-
Age influences the olfactory profiles of the migratory oriental armyworm mythimna separate at the molecular level.BMC Genomics. 2017 Jan 5;18(1):32. doi: 10.1186/s12864-016-3427-2. BMC Genomics. 2017. PMID: 28056777 Free PMC article.
-
Comparison of transcriptomes from two chemosensory organs in four decapod crustaceans reveals hundreds of candidate chemoreceptor proteins.PLoS One. 2020 Mar 12;15(3):e0230266. doi: 10.1371/journal.pone.0230266. eCollection 2020. PLoS One. 2020. PMID: 32163507 Free PMC article.
-
Distinct combinations of variant ionotropic glutamate receptors mediate thermosensation and hygrosensation in Drosophila.Elife. 2016 Sep 22;5:e17879. doi: 10.7554/eLife.17879. Elife. 2016. PMID: 27656904 Free PMC article.
References
-
- Andersson M. N., Grosse-Wilde E., Keeling C. I., Bengtsson J. M., Yuen M. M., Li M., et al. . (2013). Antennal transcriptome analysis of the chemosensory gene families in the tree killing bark beetles, Ips typographus and Dendroctonus ponderosae (Coleoptera: Curculionidae: Scolytinae). BMC Genom. 14:198. 10.1186/1471-2164-14-198 - DOI - PMC - PubMed
-
- Angerer L. M., Angerer R. C. (1992). In situ hybridization to cellular RNA with radiolabeled RNA probes, in In Situ Hybridization, ed Wilkinson D. G. (Oxford: IRL Press; ), 15–32.
-
- Bliss D. E., Mantel L. H. (1968). Adaptations of crustaceans to land - a summary and analysis of new findings. Am. Zool. 8, 673–685.
LinkOut - more resources
Full Text Sources
Other Literature Sources

