The role of cAMP in synaptic homeostasis in response to environmental temperature challenges and hyperexcitability mutations
- PMID: 25698925
- PMCID: PMC4313691
- DOI: 10.3389/fncel.2015.00010
The role of cAMP in synaptic homeostasis in response to environmental temperature challenges and hyperexcitability mutations
Abstract
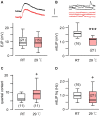
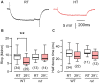
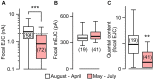
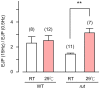
Homeostasis is the ability of physiological systems to regain functional balance following environment or experimental insults and synaptic homeostasis has been demonstrated in various species following genetic or pharmacological disruptions. Among environmental challenges, homeostatic responses to temperature extremes are critical to animal survival under natural conditions. We previously reported that axon terminal arborization in Drosophila larval neuromuscular junctions (NMJs) is enhanced at elevated temperatures; however, the amplitude of excitatory junctional potentials (EJPs) remains unaltered despite the increase in synaptic bouton numbers. Here we determine the cellular basis of this homeostatic adjustment in larvae reared at high temperature (HT, 29°C). We found that synaptic current focally recorded from individual synaptic boutons was unaffected by rearing temperature (<15°C to >30°C). However, HT rearing decreased the quantal size (amplitude of spontaneous miniature EJPs, or mEJPs), which compensates for the increased number of synaptic releasing sites to retain a normal EJP size. The quantal size decrease is accounted for by a decrease in input resistance of the postsynaptic muscle fiber, indicating an increase in membrane area that matches the synaptic growth at HT. Interestingly, a mutation in rutabaga (rut) encoding adenylyl cyclase (AC) exhibited no obvious changes in quantal size or input resistance of postsynaptic muscle cells after HT rearing, suggesting an important role for rut AC in temperature-induced synaptic homeostasis in Drosophila. This extends our previous finding of rut-dependent synaptic homeostasis in hyperexcitable mutants, e.g., slowpoke (slo). In slo larvae, the lack of BK channel function is partially ameliorated by upregulation of presynaptic Shaker (Sh) IA current to limit excessive transmitter release in addition to postsynaptic glutamate receptor recomposition that reduces the quantal size.
Keywords: input resistance; quantal content; quantal size; rutabaga adenylyl cyclase; synaptic growth.
Figures



Similar articles
-
Distinct roles of Drosophila cacophony and Dmca1D Ca(2+) channels in synaptic homeostasis: genetic interactions with slowpoke Ca(2+) -activated BK channels in presynaptic excitability and postsynaptic response.Dev Neurobiol. 2014 Jan;74(1):1-15. doi: 10.1002/dneu.22120. Epub 2013 Oct 7. Dev Neurobiol. 2014. PMID: 23959639 Free PMC article.
-
Pre- and post-synaptic mechanisms of synaptic strength homeostasis revealed by slowpoke and shaker K+ channel mutations in Drosophila.Neuroscience. 2008 Jul 17;154(4):1283-96. doi: 10.1016/j.neuroscience.2008.04.043. Epub 2008 May 2. Neuroscience. 2008. PMID: 18539401 Free PMC article.
-
Role of rut adenylyl cyclase in the ensemble regulation of presynaptic terminal excitability: reduced synaptic strength and precision in a Drosophila memory mutant.J Neurogenet. 2009;23(1-2):185-99. doi: 10.1080/01677060802471726. Epub 2008 Dec 19. J Neurogenet. 2009. PMID: 19101836 Free PMC article.
-
The synaptic bouton acts like a salt shaker.Cell Biochem Biophys. 2004;41(2):259-64. doi: 10.1385/cbb:41:2:259. Cell Biochem Biophys. 2004. PMID: 15475612 Review.
-
Presynaptic quantal plasticity: Katz's original hypothesis revisited.Synapse. 2003 Mar;47(3):184-99. doi: 10.1002/syn.10161. Synapse. 2003. PMID: 12494401 Review.
Cited by
-
The Maintenance of Synaptic Homeostasis at the Drosophila Neuromuscular Junction Is Reversible and Sensitive to High Temperature.eNeuro. 2017 Dec 12;4(6):ENEURO.0220-17.2017. doi: 10.1523/ENEURO.0220-17.2017. eCollection 2017 Nov-Dec. eNeuro. 2017. PMID: 29255795 Free PMC article.
-
Editorial: Homeostatic and retrograde signaling mechanisms modulating presynaptic function and plasticity.Front Cell Neurosci. 2015 Sep 30;9:380. doi: 10.3389/fncel.2015.00380. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26483634 Free PMC article. No abstract available.
-
Unraveling Synaptic GCaMP Signals: Differential Excitability and Clearance Mechanisms Underlying Distinct Ca2+ Dynamics in Tonic and Phasic Excitatory, and Aminergic Modulatory Motor Terminals in Drosophila.eNeuro. 2018 Feb 19;5(1):ENEURO.0362-17.2018. doi: 10.1523/ENEURO.0362-17.2018. eCollection 2018 Jan-Feb. eNeuro. 2018. PMID: 29464198 Free PMC article.
-
The effects of bacterial endotoxin LPS on synaptic transmission at the neuromuscular junction.Heliyon. 2019 Mar 28;5(3):e01430. doi: 10.1016/j.heliyon.2019.e01430. eCollection 2019 Mar. Heliyon. 2019. PMID: 30976700 Free PMC article.
-
A comparison of three different methods of eliciting rapid activity-dependent synaptic plasticity at the Drosophila NMJ.PLoS One. 2021 Nov 30;16(11):e0260553. doi: 10.1371/journal.pone.0260553. eCollection 2021. PLoS One. 2021. PMID: 34847197 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

