Systemic inflammation and the brain: novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration
- PMID: 25698933
- PMCID: PMC4313590
- DOI: 10.3389/fncel.2015.00028
Systemic inflammation and the brain: novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration
Abstract
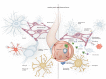
The nervous and immune systems have evolved in parallel from the early bilaterians, in which innate immunity and a central nervous system (CNS) coexisted for the first time, to jawed vertebrates and the appearance of adaptive immunity. The CNS feeds from, and integrates efferent signals in response to, somatic and autonomic sensory information. The CNS receives input also from the periphery about inflammation and infection. Cytokines, chemokines, and damage-associated soluble mediators of systemic inflammation can also gain access to the CNS via blood flow. In response to systemic inflammation, those soluble mediators can access directly through the circumventricular organs, as well as open the blood-brain barrier. The resulting translocation of inflammatory mediators can interfere with neuronal and glial well-being, leading to a break of balance in brain homeostasis. This in turn results in cognitive and behavioral manifestations commonly present during acute infections - including anorexia, malaise, depression, and decreased physical activity - collectively known as the sickness behavior (SB). While SB manifestations are transient and self-limited, under states of persistent systemic inflammatory response the cognitive and behavioral changes can become permanent. For example, cognitive decline is almost universal in sepsis survivors, and a common finding in patients with systemic lupus erythematosus. Here, we review recent genetic evidence suggesting an association between neurodegenerative disorders and persistent immune activation; clinical and experimental evidence indicating previously unidentified immune-mediated pathways of neurodegeneration; and novel immunomodulatory targets and their potential relevance for neurodegenerative disorders.
Keywords: HMGB1; TNF; anti-brain antibodies; autoimmune disorders; connectome; neurodegeneration; systemic inflammation and sepsis.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

