Alterations in neuronal activity in basal ganglia-thalamocortical circuits in the parkinsonian state
- PMID: 25698937
- PMCID: PMC4318426
- DOI: 10.3389/fnana.2015.00005
Alterations in neuronal activity in basal ganglia-thalamocortical circuits in the parkinsonian state
Abstract
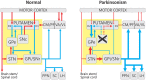
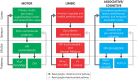
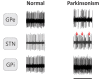
In patients with Parkinson's disease and in animal models of this disorder, neurons in the basal ganglia and related regions in thalamus and cortex show changes that can be recorded by using electrophysiologic single-cell recording techniques, including altered firing rates and patterns, pathologic oscillatory activity and increased inter-neuronal synchronization. In addition, changes in synaptic potentials or in the joint spiking activities of populations of neurons can be monitored as alterations in local field potentials (LFPs), electroencephalograms (EEGs) or electrocorticograms (ECoGs). Most of the mentioned electrophysiologic changes are probably related to the degeneration of diencephalic dopaminergic neurons, leading to dopamine loss in the striatum and other basal ganglia nuclei, although degeneration of non-dopaminergic cell groups may also have a role. The altered electrical activity of the basal ganglia and associated nuclei may contribute to some of the motor signs of the disease. We here review the current knowledge of the electrophysiologic changes at the single cell level, the level of local populations of neural elements, and the level of the entire basal ganglia-thalamocortical network in parkinsonism, and discuss the possible use of this information to optimize treatment approaches to Parkinson's disease, such as deep brain stimulation (DBS) therapy.
Keywords: LFP; Parkinson’s disease; animal models; basal ganglia; electrophysiology; extracellular recording; parkinsonism.
Figures
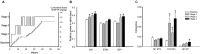
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

