Dynamics of networks during absence seizure's on- and offset in rodents and man
- PMID: 25698972
- PMCID: PMC4318340
- DOI: 10.3389/fphys.2015.00016
Dynamics of networks during absence seizure's on- and offset in rodents and man
Abstract
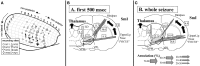
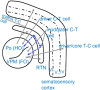
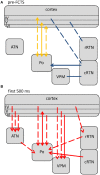
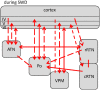
Network mechanisms relevant for the generation, maintenance and termination of spike-wave discharges (SWD), the neurophysiological hallmark of absence epilepsy, are still enigmatic and widely discussed. Within the last years, however, improvements in signal analytical techniques, applied to both animal and human fMRI, EEG, MEG, and ECoG data, greatly increased our understanding and challenged several, dogmatic concepts of SWD. This review will summarize these recent data, demonstrating that SWD are not primary generalized, are not sudden and unpredictable events. It will disentangle different functional contributions of structures within the cortico-thalamo-cortical system, relevant for the generation, generalization, maintenance, and termination of SWD and will present a new "network based" scenario for these oscillations. Similarities and differences between rodent and human data are presented demonstrating that in both species a local cortical onset zone of SWD exists, although with different locations; that in both some forms of cortical and thalamic precursor activity can be found, and that SWD occur through repetitive cyclic activity between cortex and thalamus. The focal onset zone in human data could differ between patients with varying spatial and temporal dynamics; in rats the latter is still poorly investigated.
Keywords: Granger causality; childhood absence epilepsy; cortico-thalamo-cortical system; genetic absence models; network interactions; non-linear-association analysis; pairwise-phase-consistency; seizure dynamics.
Figures
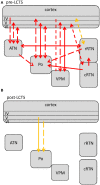
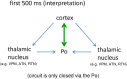

Similar articles
-
Termination of ongoing spike-wave discharges investigated by cortico-thalamic network analyses.Neurobiol Dis. 2014 Oct;70:127-37. doi: 10.1016/j.nbd.2014.06.007. Epub 2014 Jun 18. Neurobiol Dis. 2014. PMID: 24953875
-
Dynamics of directional coupling underlying spike-wave discharges.Neuroscience. 2016 Feb 9;314:75-89. doi: 10.1016/j.neuroscience.2015.11.044. Epub 2015 Dec 2. Neuroscience. 2016. PMID: 26633265
-
Higher-order thalamic nuclei facilitate the generalization and maintenance of spike-and-wave discharges of absence seizures.Neurobiol Dis. 2023 Mar;178:106025. doi: 10.1016/j.nbd.2023.106025. Epub 2023 Jan 31. Neurobiol Dis. 2023. PMID: 36731682
-
Thalamo-cortical mechanisms of sleep spindles and spike-wave discharges in rat model of absence epilepsy (a review).Epilepsy Res. 2010 Mar;89(1):17-26. doi: 10.1016/j.eplepsyres.2009.09.005. Epub 2009 Oct 13. Epilepsy Res. 2010. PMID: 19828296 Review.
-
Genetic absence epilepsy in rats from Strasbourg--a review.J Neural Transm Suppl. 1992;35:37-69. doi: 10.1007/978-3-7091-9206-1_4. J Neural Transm Suppl. 1992. PMID: 1512594 Review.
Cited by
-
NMDA Receptor Expression in the Thalamus of the Stargazer Model of Absence Epilepsy.Sci Rep. 2017 Feb 21;7:42926. doi: 10.1038/srep42926. Sci Rep. 2017. PMID: 28220891 Free PMC article.
-
CaV3.2 calcium channels control NMDA receptor-mediated transmission: a new mechanism for absence epilepsy.Genes Dev. 2015 Jul 15;29(14):1535-51. doi: 10.1101/gad.260869.115. Genes Dev. 2015. PMID: 26220996 Free PMC article.
-
Absence Seizure Control by a Brain Computer Interface.Sci Rep. 2017 May 29;7(1):2487. doi: 10.1038/s41598-017-02626-y. Sci Rep. 2017. PMID: 28555070 Free PMC article.
-
Dynamical mesoscale model of absence seizures in genetic models.PLoS One. 2020 Sep 29;15(9):e0239125. doi: 10.1371/journal.pone.0239125. eCollection 2020. PLoS One. 2020. PMID: 32991590 Free PMC article.
-
Progressive, Seizure-Like, Spike-Wave Discharges Are Common in Both Injured and Uninjured Sprague-Dawley Rats: Implications for the Fluid Percussion Injury Model of Post-Traumatic Epilepsy.J Neurosci. 2015 Jun 17;35(24):9194-204. doi: 10.1523/JNEUROSCI.0919-15.2015. J Neurosci. 2015. PMID: 26085641 Free PMC article.
References
-
- Aker R. G., Ozyurt H. B., Yananli H. R., Cakmak Y. O., Ozkaynakci A. E., Sehirli U., et al. . (2006). GABA(A) receptor mediated transmission in the thalamic reticular nucleus of rats with genetic absence epilepsy shows regional differences: functional implications. Brain Res. 1111, 213–221. 10.1016/j.brainres.2006.06.118 - DOI - PubMed
-
- Avanzini G., De Curtis M., Marescaux C., Panzica F., Spreafico R., Vergnes M. (1992). Role of the thalamic reticular nucleus in the generation of rhythmic thalamo-cortical activities subserving spike and waves. J. Neural Transm. Suppl. 35, 85–95. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

