Functional role of voltage gated Ca(2+) channels in heart automaticity
- PMID: 25698974
- PMCID: PMC4313592
- DOI: 10.3389/fphys.2015.00019
Functional role of voltage gated Ca(2+) channels in heart automaticity
Abstract
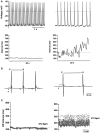
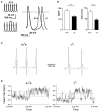
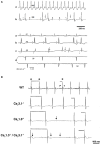
Pacemaker activity of automatic cardiac myocytes controls the heartbeat in everyday life. Cardiac automaticity is under the control of several neurotransmitters and hormones and is constantly regulated by the autonomic nervous system to match the physiological needs of the organism. Several classes of ion channels and proteins involved in intracellular Ca(2+) dynamics contribute to pacemaker activity. The functional role of voltage-gated calcium channels (VGCCs) in heart automaticity and impulse conduction has been matter of debate for 30 years. However, growing evidence shows that VGCCs are important regulators of the pacemaker mechanisms and play also a major role in atrio-ventricular impulse conduction. Incidentally, studies performed in genetically modified mice lacking L-type Cav1.3 (Cav1.3(-/-)) or T-type Cav3.1 (Cav3.1(-/-)) channels show that genetic inactivation of these channels strongly impacts pacemaking. In cardiac pacemaker cells, VGCCs activate at negative voltages at the beginning of the diastolic depolarization and importantly contribute to this phase by supplying inward current. Loss-of-function of these channels also impairs atrio-ventricular conduction. Furthermore, inactivation of Cav1.3 channels promotes also atrial fibrillation and flutter in knockout mice suggesting that these channels can play a role in stabilizing atrial rhythm. Genomic analysis demonstrated that Cav1.3 and Cav3.1 channels are widely expressed in pacemaker tissue of mice, rabbits and humans. Importantly, human diseases of pacemaker activity such as congenital bradycardia and heart block have been attributed to loss-of-function of Cav1.3 and Cav3.1 channels. In this article, we will review the current knowledge on the role of VGCCs in the generation and regulation of heart rate and rhythm. We will discuss also how loss of Ca(2+) entry through VGCCs could influence intracellular Ca(2+) handling and promote atrial arrhythmias.
Keywords: L-type Ca2+ channel; T-type Ca2+ channels; atrioventricular node; heart automaticity; sinoatrial node.
Figures

Similar articles
-
Concomitant genetic ablation of L-type Cav1.3 (α1D) and T-type Cav3.1 (α1G) Ca2+ channels disrupts heart automaticity.Sci Rep. 2020 Nov 3;10(1):18906. doi: 10.1038/s41598-020-76049-7. Sci Rep. 2020. PMID: 33144668 Free PMC article.
-
T-type channels in the sino-atrial and atrioventricular pacemaker mechanism.Pflugers Arch. 2014 Apr;466(4):791-9. doi: 10.1007/s00424-014-1482-6. Epub 2014 Feb 27. Pflugers Arch. 2014. PMID: 24573175 Review.
-
Channelopathies of voltage-gated L-type Cav1.3/α1D and T-type Cav3.1/α1G Ca2+ channels in dysfunction of heart automaticity.Pflugers Arch. 2020 Jul;472(7):817-830. doi: 10.1007/s00424-020-02421-1. Epub 2020 Jun 29. Pflugers Arch. 2020. PMID: 32601767 Review.
-
L-type Cav1.3 channels regulate ryanodine receptor-dependent Ca2+ release during sino-atrial node pacemaker activity.Cardiovasc Res. 2016 Mar 1;109(3):451-61. doi: 10.1093/cvr/cvw006. Epub 2016 Jan 19. Cardiovasc Res. 2016. PMID: 26786159
-
Functional roles of Ca(v)1.3, Ca(v)3.1 and HCN channels in automaticity of mouse atrioventricular cells: insights into the atrioventricular pacemaker mechanism.Channels (Austin). 2011 May-Jun;5(3):251-61. doi: 10.4161/chan.5.3.15266. Epub 2011 May 1. Channels (Austin). 2011. PMID: 21406960 Free PMC article.
Cited by
-
Eps15 Homology Domain-containing Protein 3 Regulates Cardiac T-type Ca2+ Channel Targeting and Function in the Atria.J Biol Chem. 2015 May 8;290(19):12210-21. doi: 10.1074/jbc.M115.646893. Epub 2015 Mar 30. J Biol Chem. 2015. PMID: 25825486 Free PMC article.
-
Cohesin-protein Shugoshin-1 controls cardiac automaticity via HCN4 pacemaker channel.Nat Commun. 2021 May 5;12(1):2551. doi: 10.1038/s41467-021-22737-5. Nat Commun. 2021. PMID: 33953173 Free PMC article.
-
Murine Electrophysiological Models of Cardiac Arrhythmogenesis.Physiol Rev. 2017 Jan;97(1):283-409. doi: 10.1152/physrev.00007.2016. Physiol Rev. 2017. PMID: 27974512 Free PMC article. Review.
-
Physiological Roles of the Rapidly Activated Delayed Rectifier K+ Current in Adult Mouse Heart Primary Pacemaker Activity.Int J Mol Sci. 2021 Apr 30;22(9):4761. doi: 10.3390/ijms22094761. Int J Mol Sci. 2021. PMID: 33946248 Free PMC article.
-
Inherited and Acquired Rhythm Disturbances in Sick Sinus Syndrome, Brugada Syndrome, and Atrial Fibrillation: Lessons from Preclinical Modeling.Cells. 2021 Nov 15;10(11):3175. doi: 10.3390/cells10113175. Cells. 2021. PMID: 34831398 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

