The archaellum: how Archaea swim
- PMID: 25699024
- PMCID: PMC4307647
- DOI: 10.3389/fmicb.2015.00023
The archaellum: how Archaea swim
Abstract
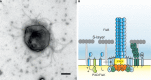
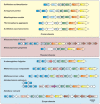
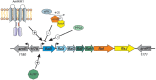
Recent studies on archaeal motility have shown that the archaeal motility structure is unique in several aspects. Although it fulfills the same swimming function as the bacterial flagellum, it is evolutionarily and structurally related to the type IV pilus. This was the basis for the recent proposal to term the archaeal motility structure the "archaellum." This review illustrates the key findings that led to the realization that the archaellum was a novel motility structure and presents the current knowledge about the structural composition, mechanism of assembly and regulation, and the posttranslational modifications of archaella.
Keywords: archaeal flagellum; archaellum; motility; motor complex; type IV pili.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

