The Jekyll and Hyde story of IL17-Producing γδT Cells
- PMID: 25699053
- PMCID: PMC4316782
- DOI: 10.3389/fimmu.2015.00037
The Jekyll and Hyde story of IL17-Producing γδT Cells
Abstract
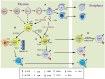
In comparison to conventional αβT cells, γδT cells are considered as specialized T cells based on their contributions in regulating immune response. γδT cells sense early environmental signals and initiate local immune-surveillance. The development of functional subtypes of γδT cells takes place in the thymus but they also exhibit plasticity in response to the activating signals and cytokines encountered in the extrathymic region. Thymic development of Tγδ1 requires strong TCR, CD27, and Skint-1 signals. However, differentiation of IL17-producing γδT cells (Tγδ17) is independent of Skint-1 or CD27 but requires notch signaling along with IL6 and TGFβ cytokines in the presence of weak TCR signal. In response to cytokines like IL23, IL6, and IL1β, Tγδ17 outshine Th17 cells for early activation and IL17 secretion. Despite expressing similar repertoire of lineage transcriptional factors, cytokines, and chemokine receptors, Tγδ17 cells differ from Th17 in spatial and temporal fashion. There are compelling reasons to consider significant role of Tγδ17 cells in regulating inflammation and thereby disease outcome. Tγδ17 cells regulate mobilization of innate immune cells and induce keratinocytes to secrete anti-microbial peptides thus exhibiting protective functions in anti-microbial immunity. In contrast, dysregulated Tγδ17 cells inhibit Treg cells, exacerbate autoimmunity, and are also known to support carcinogenesis by enhancing angiogenesis. The mechanism associated with this dual behavior of Tγδ17 is not clear. To exploit, Tγδ17 cells for beneficial use requires comprehensive analysis of their biology. Here, we summarize the current understanding on the characteristics, development, and functions of Tγδ17 cells in various pathological scenarios.
Keywords: IL17; Tγδ17; cancer; infection; inflammation; γδT cell.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

