DNA damage response and evasion from immunosurveillance in CLL: new options for NK cell-based immunotherapies
- PMID: 25699074
- PMCID: PMC4316781
- DOI: 10.3389/fgene.2015.00011
DNA damage response and evasion from immunosurveillance in CLL: new options for NK cell-based immunotherapies
Abstract
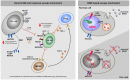
Chronic lymphocytic leukemia (CLL) is the most prominent B cell malignancy among adults in the Western world and characterized by a clonal expansion of B cells. The patients suffer from severe immune defects resulting in increased susceptibility to infections and failure to generate an antitumor immune response. Defects in both, DNA damage response (DDR) pathway and crosstalk with the tissue microenvironment have been reported to play a crucial role for the survival of CLL cells, therapy resistance and impaired immune response. To this end, major advances over the past years have highlighted several T cell immune evasion mechanisms in CLL. Here, we discuss the consequences of an impaired DDR pathway for detection and elimination of CLL cells by natural killer (NK) cells. NK cells are considered to be a major component of the immunosurveillance in leukemia but NK cell activity is impaired in CLL. Restoration of NK cell activity using immunoligands and immunoconstructs in combination with the conventional chemotherapy may provide a future perspective for CLL treatment.
Keywords: DNA damage response; chronic lymphocytic leukemia; immunoconstructs; immunoligands; immunotherapy; natural killer cell.
Figures

References
-
- Austen B., Skowronska A., Baker C., Powell J. E., Gardiner A., Oscier D., et al. (2007). Mutation status of the residual ATM allele is an important determinant of the cellular response to chemotherapy and survival in patients with chronic lymphocytic leukemia containing an 11q deletion. J. Clin. Oncol. 25, 5448–5457. 10.1200/JCO.2007.11.2649 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

