ErbB polymorphisms: insights and implications for response to targeted cancer therapeutics
- PMID: 25699077
- PMCID: PMC4316710
- DOI: 10.3389/fgene.2015.00017
ErbB polymorphisms: insights and implications for response to targeted cancer therapeutics
Abstract
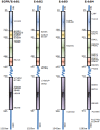
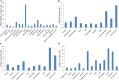
Advances in high-throughput genomic-scanning have expanded the repertory of genetic variations in DNA sequences encoding ErbB tyrosine kinase receptors in humans, including single nucleotide polymorphisms (SNPs), polymorphic repetitive elements, microsatellite variations, small-scale insertions and deletions. The ErbB family members: EGFR, ErbB2, ErbB3, and ErbB4 receptors are established as drivers of many aspects of tumor initiation and progression to metastasis. This knowledge has provided rationales for the development of an arsenal of anti-ErbB therapeutics, ranging from small molecule kinase inhibitors to monoclonal antibodies. Anti-ErbB agents are becoming the cornerstone therapeutics for the management of cancers that overexpress hyperactive variants of ErbB receptors, in particular ErbB2-positive breast cancer and non-small cell lung carcinomas. However, their clinical benefit has been limited to a subset of patients due to a wide heterogeneity in drug response despite the expression of the ErbB targets, attributed to intrinsic (primary) and to acquired (secondary) resistance. Somatic mutations in ErbB tyrosine kinase domains have been extensively investigated in preclinical and clinical setting as determinants for either high sensitivity or resistance to anti-ErbB therapeutics. In contrast, only scant information is available on the impact of SNPs, which are widespread in genes encoding ErbB receptors, on receptor structure and activity, and their predictive values for drug susceptibility. This review aims to briefly update polymorphic variations in genes encoding ErbB receptors based on recent advances in deep sequencing technologies, and to address challenging issues for a better understanding of the functional impact of single versus combined SNPs in ErbB genes to receptor topology, receptor-drug interaction, and drug susceptibility. The potential of exploiting SNPs in the era of stratified targeted therapeutics is discussed.
Keywords: ErbB receptors; SNPs; anti-ErbB therapeutics; cancer; drug response; resistance.
Figures
References
-
- Amiri-Kordestani L., Wedam S., Zhang L., Tang S., Tilley A., Ibrahim A., et al. (2014). First FDA Approval of neoadjuvant therapy for breast cancer: pertuzumab for the treatment of patients with HER2-positive breast cancer. Clin. Cancer Res. 20, 5359–5364. 10.1158/1078-0432.CCR-14-1268 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

