Sativex in the management of multiple sclerosis-related spasticity: role of the corticospinal modulation
- PMID: 25699191
- PMCID: PMC4325203
- DOI: 10.1155/2015/656582
Sativex in the management of multiple sclerosis-related spasticity: role of the corticospinal modulation
Abstract
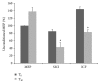
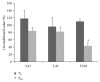
Sativex is an emergent treatment option for spasticity in patients affected by multiple sclerosis (MS). This oromucosal spray, acting as a partial agonist at cannabinoid receptors, may modulate the balance between excitatory and inhibitory neurotransmitters, leading to muscle relaxation that is in turn responsible for spasticity improvement. Nevertheless, since the clinical assessment may not be sensitive enough to detect spasticity changes, other more objective tools should be tested to better define the real drug effect. The aim of our study was to investigate the role of Sativex in improving spasticity and related symptomatology in MS patients by means of an extensive neurophysiological assessment of sensory-motor circuits. To this end, 30 MS patients underwent a complete clinical and neurophysiological examination, including the following electrophysiological parameters: motor threshold, motor evoked potentials amplitude, intracortical excitability, sensory-motor integration, and Hmax/Mmax ratio. The same assessment was applied before and after one month of continuous treatment. Our data showed an increase of intracortical inhibition, a significant reduction of spinal excitability, and an improvement in spasticity and associated symptoms. Thus, we can speculate that Sativex could be effective in reducing spasticity by means of a double effect on intracortical and spinal excitability.
Figures
References
-
- Beard S., Hunn A., Wight J. Treatments for spasticity and pain in multiple sclerosis: a systematic review. Health Technology Assessment. 2003;7:1–11. - PubMed
-
- Novotna A., Mares J., Ratcliffe S., et al. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols (Sativex), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. European Journal of Neurology. 2011;18(9):1122–1131. doi: 10.1111/j.1468-1331.2010.03328.x. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical