Internal and External Feedback Circuits for Skilled Forelimb Movement
- PMID: 25699987
- PMCID: PMC4475648
- DOI: 10.1101/sqb.2014.79.024786
Internal and External Feedback Circuits for Skilled Forelimb Movement
Abstract
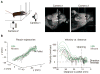
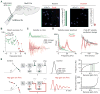
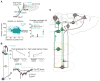
Skilled motor behavior emerges from interactions between efferent neural pathways that induce muscle contraction and feedback systems that report and refine movement. Two broad classes of feedback projections modify motor output, one from the periphery and a second that originates within the central nervous system. The mechanisms through which these pathways influence movement remain poorly understood, however. Here we discuss recent studies that delineate spinal circuitry that binds external and internal feedback pathways to forelimb motor behavior. A spinal presynaptic inhibitory circuit regulates the strength of external feedback, promoting limb stability during goal-directed reaching. A distinct excitatory propriospinal circuit conveys copies of motor commands to the cerebellum, establishing an internal feedback loop that rapidly modulates forelimb motor output. The behavioral consequences of manipulating these two circuits reveal distinct controls on motor performance and provide an initial insight into feedback strategies that underlie skilled forelimb movement.
Copyright © 2014 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures



References
-
- Ali MS, Hou ZK, Noori MN. Stability and performance of feedback control systems with time delays. Computers & Structures. 1998;66:241–248.
-
- Alstermark B, Isa T. Circuits for skilled reaching and grasping. Annual review of neuroscience. 2012;35:559–578. - PubMed
-
- Alstermark B, Kummel H, Pinter MJ, Tantisira B. Integration in descending motor pathways controlling the forelimb in the cat. 17. Axonal projection and termination of C3-C4 propriospinal neurones in the C6-Th1 segments. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 1990;81:447–461. - PubMed
-
- Alstermark B, Lindstrom S, Lundberg A, Sybirska E. Integration in descending motor pathways controlling the forelimb in the cat. 8. Ascending projection to the lateral reticular nucleus from C3-C4 propriospinal also projecting to forelimb motoneurones. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 1981a;42:282–298. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
