Comparative proteomic and biochemical analyses reveal different molecular events occurring in the process of fiber initiation between wild-type allotetraploid cotton and its fuzzless-lintless mutant
- PMID: 25700002
- PMCID: PMC4336136
- DOI: 10.1371/journal.pone.0117049
Comparative proteomic and biochemical analyses reveal different molecular events occurring in the process of fiber initiation between wild-type allotetraploid cotton and its fuzzless-lintless mutant
Abstract
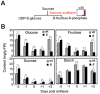
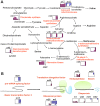
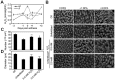
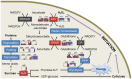
To explore lint fiber initiation-related proteins in allotetraploid cotton (Gossypium hirsutum L.), a comparative proteomic analysis was performed between wild-type cotton (Xu-142) and its fuzzless-lintless mutant (Xu-142-fl) at five developmental time points for lint fiber initiation from -3 to +3 days post-anthesis (dpa). Using two-dimensional gel electrophoresis (2-DE) combined with mass spectrometry (MS) analyses, 91 differentially accumulated protein (DAP) species that are related to fiber initiation were successfully identified, of which 58 preferentially accumulated in the wild-type and 33 species in the fl mutant. These DAPs are involved in various cellular and metabolic processes, mainly including important energy/carbohydrate metabolism, redox homeostasis, amino acid and fatty acid biosynthesis, protein quality control, cytoskeleton dynamics, and anthocyanidin metabolism. Further physiological and biochemical experiments revealed dynamic changes in the carbohydrate flux and H2O2 levels in the cotton fiber initiation process. Compared with those in the fl mutant, the contents of glucose and fructose in wild-type ovules sharply increased after anthesis with a relatively higher rate of amino acid biosynthesis. The relative sugar starvation and lower rate of amino acid biosynthesis in the fl mutant ovules may impede the carbohydrate/energy supply and cell wall synthesis, which is consistent with the proteomic results. However, the H2O2 burst was only observed in the wild-type ovules on the day of anthesis. Cotton boll injection experiments in combination with electron microscope observation collectively indicated that H2O2 burst, which is negatively regulated by ascorbate peroxidases (APx), plays an important role in the fiber initiation process. Taken together, our study demonstrates a putative network of DAP species related to fiber initiation in cotton ovules and provides a foundation for future studies on the specific functions of these proteins in fiber development.
Conflict of interest statement
Figures



Similar articles
-
Functional genomics of fuzzless-lintless mutant of Gossypium hirsutum L. cv. MCU5 reveal key genes and pathways involved in cotton fibre initiation and elongation.BMC Genomics. 2012 Nov 14;13:624. doi: 10.1186/1471-2164-13-624. BMC Genomics. 2012. PMID: 23151214 Free PMC article.
-
Comparative proteomic analysis reveals differentially expressed proteins correlated with fuzz fiber initiation in diploid cotton (Gossypium arboreum L.).J Proteomics. 2013 Apr 26;82:113-29. doi: 10.1016/j.jprot.2013.02.020. Epub 2013 Mar 6. J Proteomics. 2013. PMID: 23474080
-
Comparative proteomic analysis reveals the mechanisms governing cotton fiber differentiation and initiation.J Proteomics. 2012 Jan 4;75(3):845-56. doi: 10.1016/j.jprot.2011.09.025. Epub 2011 Oct 8. J Proteomics. 2012. PMID: 22015716
-
Cotton proteomics for deciphering the mechanism of environment stress response and fiber development.J Proteomics. 2014 Jun 13;105:74-84. doi: 10.1016/j.jprot.2014.03.017. Epub 2014 Mar 27. J Proteomics. 2014. PMID: 24680693 Review.
-
Understanding the role of phytohormones in cotton fiber development through omic approaches; recent advances and future directions.Int J Biol Macromol. 2020 Nov 15;163:1301-1313. doi: 10.1016/j.ijbiomac.2020.07.104. Epub 2020 Jul 15. Int J Biol Macromol. 2020. PMID: 32679330 Review.
Cited by
-
GhMCS1, the Cotton Orthologue of Human GRIM-19, Is a Subunit of Mitochondrial Complex I and Associated with Cotton Fibre Growth.PLoS One. 2016 Sep 15;11(9):e0162928. doi: 10.1371/journal.pone.0162928. eCollection 2016. PLoS One. 2016. PMID: 27632161 Free PMC article.
-
Two-Dimensional Gel Electrophoresis-Based Proteomic Analysis Reveals N-terminal Truncation of the Hsc70 Protein in Cotton Fibers In Vivo.Sci Rep. 2016 Nov 11;6:36961. doi: 10.1038/srep36961. Sci Rep. 2016. PMID: 27833127 Free PMC article.
-
Comparative Proteomic Analysis of Cotton Fiber Development and Protein Extraction Method Comparison in Late Stage Fibers.Proteomes. 2016 Feb 3;4(1):7. doi: 10.3390/proteomes4010007. Proteomes. 2016. PMID: 28248216 Free PMC article.
-
Systematically and Comprehensively Understanding the Regulation of Cotton Fiber Initiation: A Review.Plants (Basel). 2023 Nov 4;12(21):3771. doi: 10.3390/plants12213771. Plants (Basel). 2023. PMID: 37960127 Free PMC article. Review.
-
Long non-coding RNAs and their potential functions in Ligon-lintless-1 mutant cotton during fiber development.BMC Genomics. 2019 Aug 19;20(1):661. doi: 10.1186/s12864-019-5978-5. BMC Genomics. 2019. PMID: 31426741 Free PMC article.
References
-
- Wilkins T, Arpat A, Sickler B (2005) Cotton fiber genomics: Developmental mechanisms. Pflanzenschutz-Nachrichten Bayer 58: 119–139.
-
- Wu YR, Adriane CM, Rosemary GW, Llewellyn DJ, Dennis ES (2006) Expression profiling identifies genes expressed early during lint fibre initiation in cotton. Plant Cell Physiol 47: 107–127. - PubMed
-
- Turley RB, Kloth RH (2002) Identification of a third fuzzless seed locus in upland cotton (Gossypium hirsutum L.). J Hered 93: 359–364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

