Dynamical transitions in a pollination-herbivory interaction: a conflict between mutualism and antagonism
- PMID: 25700003
- PMCID: PMC4336290
- DOI: 10.1371/journal.pone.0117964
Dynamical transitions in a pollination-herbivory interaction: a conflict between mutualism and antagonism
Abstract
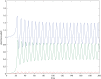
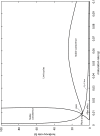
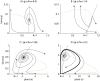
Plant-pollinator associations are often seen as purely mutualistic, while in reality they can be more complex. Indeed they may also display a diverse array of antagonistic interactions, such as competition and victim-exploiter interactions. In some cases mutualistic and antagonistic interactions are carried-out by the same species but at different life-stages. As a consequence, population structure affects the balance of inter-specific associations, a topic that is receiving increased attention. In this paper, we developed a model that captures the basic features of the interaction between a flowering plant and an insect with a larval stage that feeds on the plant's vegetative tissues (e.g. leaves) and an adult pollinator stage. Our model is able to display a rich set of dynamics, the most remarkable of which involves victim-exploiter oscillations that allow plants to attain abundances above their carrying capacities and the periodic alternation between states dominated by mutualism or antagonism. Our study indicates that changes in the insect's life cycle can modify the balance between mutualism and antagonism, causing important qualitative changes in the interaction dynamics. These changes in the life cycle could be caused by a variety of external drivers, such as temperature, plant nutrients, pesticides and changes in the diet of adult pollinators.
Conflict of interest statement
Figures


Similar articles
-
The Olfactory Neuroecology of Herbivory, Hostplant Selection and Plant-Pollinator Interactions.Integr Comp Biol. 2016 Nov;56(5):856-864. doi: 10.1093/icb/icw096. Epub 2016 Jul 28. Integr Comp Biol. 2016. PMID: 27471226 Review.
-
How plants connect pollination and herbivory networks and their contribution to community stability.Ecology. 2016 Apr;97(4):908-917. doi: 10.1890/15-0132.1. Ecology. 2016. PMID: 28792600
-
Shifts in pollinator population structure may jeopardize pollination service.J Theor Biol. 2014 Jul 7;352:24-30. doi: 10.1016/j.jtbi.2014.02.030. Epub 2014 Mar 4. J Theor Biol. 2014. PMID: 24607744
-
Herbivore-induced pollinator limitation increases community stability of mutualism-antagonism continuum.Biosystems. 2023 Jul;229:104929. doi: 10.1016/j.biosystems.2023.104929. Epub 2023 May 20. Biosystems. 2023. PMID: 37217159
-
Diversification and coevolution in brood pollination mutualisms: Windows into the role of biotic interactions in generating biological diversity.Am J Bot. 2016 Oct;103(10):1783-1792. doi: 10.3732/ajb.1600056. Epub 2016 Oct 7. Am J Bot. 2016. PMID: 27765775 Free PMC article. Review.
Cited by
-
A unifying framework for interpreting and predicting mutualistic systems.Nat Commun. 2019 Jan 16;10(1):242. doi: 10.1038/s41467-018-08188-5. Nat Commun. 2019. PMID: 30651549 Free PMC article.
References
-
- Adler LS, Bronstein JL (2004) Attracting antagonists: Does floral nectar increase leaf herbivory? Ecology 85: 1519–1526. 10.1890/03-0409 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

