Metabolic and hypoxic adaptation to anti-angiogenic therapy: a target for induced essentiality
- PMID: 25700172
- PMCID: PMC4403040
- DOI: 10.15252/emmm.201404271
Metabolic and hypoxic adaptation to anti-angiogenic therapy: a target for induced essentiality
Abstract
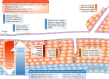
Anti-angiogenic therapy has increased the progression-free survival of many cancer patients but has had little effect on overall survival, even in colon cancer (average 6-8 weeks) due to resistance. The current licensed targeted therapies all inhibit VEGF signalling (Table 1). Many mechanisms of resistance to anti-VEGF therapy have been identified that enable cancers to bypass the angiogenic blockade. In addition, over the last decade, there has been increasing evidence for the role that the hypoxic and metabolic responses play in tumour adaptation to anti-angiogenic therapy. The hypoxic tumour response, through the transcription factor hypoxia-inducible factors (HIFs), induces major gene expression, metabolic and phenotypic changes, including increased invasion and metastasis. Pre-clinical studies combining anti-angiogenics with inhibitors of tumour hypoxic and metabolic adaptation have shown great promise, and combination clinical trials have been instigated. Understanding individual patient response and the response timing, given the opposing effects of vascular normalisation versus reduced perfusion seen with anti-angiogenics, provides a further hurdle in the paradigm of personalised therapeutic intervention. Additional approaches for targeting the hypoxic tumour microenvironment are being investigated in pre-clinical and clinical studies that have potential for producing synthetic lethality in combination with anti-angiogenic therapy as a future therapeutic strategy.
Keywords: angiogenesis; anti‐VEGF therapy; combination therapy; hypoxia; metabolism.
© 2015 The Authors. Published under the terms of the CC BY 4.0 license.
Figures

References
-
- Agani F, Jiang BH. Oxygen-independent regulation of HIF-1: novel involvement of PI3K/AKT/mTOR pathway in cancer. Curr Cancer Drug Targets. 2013;13:245–251. - PubMed
-
- Ashworth A, Lord CJ, Reis-Filho JS. Genetic interactions in cancer progression and treatment. Cell. 2011;145:30–38. - PubMed
-
- Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–177. - PubMed
-
- Batchelor TT, Gerstner ER, Emblem KE, Duda DG, Kalpathy-Cramer J, Snuderl M, Ancukiewicz M, Polaskova P, Pinho MC, Jennings D, et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci U S A. 2013;110:19059–19064. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

