Role of miR-155 in the pathogenesis of herpetic stromal keratitis
- PMID: 25700796
- PMCID: PMC4380872
- DOI: 10.1016/j.ajpath.2014.12.021
Role of miR-155 in the pathogenesis of herpetic stromal keratitis
Abstract
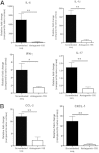
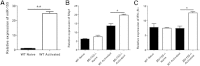
Ocular infection with herpes simplex virus 1 can result in a chronic immunoinflammatory stromal keratitis (SK) lesion that is a significant cause of human blindness. A key to controlling SK lesion severity is to identify cellular and molecular events responsible for tissue damage and to manipulate them therapeutically. Potential targets for therapy are miRNAs, but these are minimally explored especially in responses to infection. Here, we demonstrated that Mir155 expression was up-regulated after ocular herpes simplex virus 1 infection, with the increased Mir155 expression occurring mainly in macrophages and CD4(+) T cells and to a lesser extent in neutrophils. In vivo studies indicated that Mir155 knockout mice were more resistant to herpes SK with marked suppression of T helper cells type 1 and 17 responses both in the ocular lesions and the lymphoid organs. The reduced SK lesion severity was reflected by increased phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase 1 and interferon-γ receptor α-chain levels in activated CD4(+) T cells in the lymph nodes. Finally, in vivo silencing of miR-155 by the provision of antagomir-155 nanoparticles to herpes simplex virus 1-infected mice led to diminished SK lesions and corneal vascularization. In conclusion, our results indicate that miR-155 contributes to the pathogenesis of SK and represents a promising target to control SK severity.
Copyright © 2015 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Biswas P.S., Rouse B.T. Early events in HSV keratitis–setting the stage for a blinding disease. Microbes Infect. 2005;7:799–810. - PubMed
-
- Niemialtowski M.G., Rouse B.T. Predominance of Th1 cells in ocular tissues during herpetic stromal keratitis. J Immunol. 1992;149:3035–3039. - PubMed
-
- Thomas J., Gangappa S., Kanangat S., Rouse B.T. On the essential involvement of neutrophils in the immunopathologic disease: herpetic stromal keratitis. J Immunol. 1997;158:1383–1391. - PubMed
-
- Daheshia M., Kanangat S., Rouse B.T. Production of key molecules by ocular neutrophils early after herpetic infection of the cornea. Exp Eye Res. 1998;67:619–624. - PubMed
-
- Bauer D., Mrzyk S., van Rooijen N., Steuhl K.P., Heiligenhaus A. Macrophage-depletion influences the course of murine HSV-1 keratitis. Curr Eye Res. 2000;20:45–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

