Sarcolipin overexpression improves muscle energetics and reduces fatigue
- PMID: 25701006
- PMCID: PMC4398885
- DOI: 10.1152/japplphysiol.01066.2014
Sarcolipin overexpression improves muscle energetics and reduces fatigue
Abstract
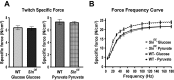
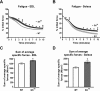
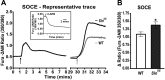
Sarcolipin (SLN) is a regulator of sarcoendoplasmic reticulum calcium ATPase in skeletal muscle. Recent studies using SLN-null mice have identified SLN as a key player in muscle thermogenesis and metabolism. In this study, we exploited a SLN overexpression (Sln(OE)) mouse model to determine whether increased SLN level affected muscle contractile properties, exercise capacity/fatigue, and metabolic rate in whole animals and isolated muscle. We found that Sln(OE) mice are more resistant to fatigue and can run significantly longer distances than wild-type (WT). Studies with isolated extensor digitorum longus (EDL) muscles showed that Sln(OE) EDL produced higher twitch force than WT. The force-frequency curves were not different between WT and Sln(OE) EDLs, but at lower frequencies the pyruvate-induced potentiation of force was significantly higher in Sln(OE) EDL. SLN overexpression did not alter the twitch and force-frequency curve in isolated soleus muscle. However, during a 10-min fatigue protocol, both EDL and soleus from Sln(OE) mice fatigued significantly less than WT muscles. Interestingly, Sln(OE) muscles showed higher carnitine palmitoyl transferase-1 protein expression, which could enhance fatty acid metabolism. In addition, lactate dehydrogenase expression was higher in Sln(OE) EDL, suggesting increased glycolytic capacity. We also found an increase in store-operated calcium entry (SOCE) in isolated flexor digitorum brevis fibers of Sln(OE) compared with WT mice. These data allow us to conclude that increased SLN expression improves skeletal muscle performance during prolonged muscle activity by increasing SOCE and muscle energetics.
Keywords: Ca2+ ATPase; muscle fatigue; muscle metabolism.
Copyright © 2015 the American Physiological Society.
Figures


References
-
- Babu GJ, Bhupathy P, Petrashevskaya NN, Wang H, Raman S, Wheeler D, Jagatheesan G, Wieczorek D, Schwartz A, Janssen PM, Ziolo MT, Periasamy M. Targeted overexpression of sarcolipin in the mouse heart decreases sarcoplasmic reticulum calcium transport and cardiac contractility. J Biol Chem 281: 3972–3979, 2006. - PubMed
-
- Babu GJ, Zheng Z, Natarajan P, Wheeler D, Janssen PM, Periasamy M. Overexpression of sarcolipin decreases myocyte contractility and calcium transient. Cardiovasc Res 65: 177–186, 2005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

