Structure-function analysis of SAP97, a modular scaffolding protein that drives dendrite growth
- PMID: 25701814
- PMCID: PMC4393785
- DOI: 10.1016/j.mcn.2015.02.011
Structure-function analysis of SAP97, a modular scaffolding protein that drives dendrite growth
Abstract
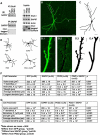
Activation of AMPA receptors assembled with the GluA1 subunit can promote dendrite growth in a manner that depends on its direct binding partner, SAP97. SAP97 is a modular scaffolding protein that has at least seven recognizable protein-protein interaction domains. Several complementary approaches were employed to show that the dendrite branching promoting action of full length SAP97 depends on ligand(s) that bind to the PDZ3 domain. Ligand(s) to PDZ1, PDZ2 and I3 domains also contribute to dendrite growth. The ability of PDZ3 ligand(s) to promote dendrite growth depends on localization at the plasma membrane along with GluA1 and SAP97. These results suggest that the assembly of a multi-protein complex at or near synapses is vital for the translation of AMPA-R activity into dendrite growth.
Keywords: GluA1; PDZ domain; Spinal cord neuron.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures





References
-
- Chen SX, Tari PK, She K, Haas K. Neurexin-neuroligin cell adhesion complexes contribute to synaptotropic dendritogenesis via growth stabilization mechanisms in vivo. Neuron. 2010;67:967–983. - PubMed
-
- Constantine-Paton M. NMDA receptor as a mediator of activity-dependent synaptogenesis in the developing brain. Cold Spring Harbor SympQuantBiol. 1990;LV:431–443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

