Leukocyte telomere length: a novel biomarker to predict the prognosis of glioma patients
- PMID: 25702101
- PMCID: PMC11823758
- DOI: 10.1007/s00432-015-1938-x
Leukocyte telomere length: a novel biomarker to predict the prognosis of glioma patients
Abstract
Purpose: Epidemiological studies have demonstrated that leukocyte telomere length is associated with the developing risk of various malignancies, including glioma. However, its prognostic value in glioma patients has never been investigated.
Methods: Relative telomere length (RTL) of peripheral blood leukocytes from 301 glioma patients were examined using a real-time PCR-based method. Kaplan-Meier curves and Cox proportional hazards regression model were used to assess the association of RTL with clinical outcomes of patients. To explore the potential mechanism, the immune phenotype of peripheral blood mononuclear cells (PBMCs) and concentrations of several cytokines from another 20 glioma patients were detected by flow cytometry and enzyme-linked immunosorbent assay (ELISA), respectively. The relationship between RTL and immunological characteristics of PBMCs were further analyzed.
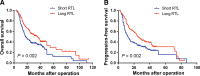
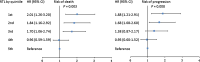
Results: Patients with short RTL showed both poorer overall survival (OS) and progression-free survival (PFS) than those with long RTL. Multivariate Cox regression analysis demonstrated that RTL was an independent prognostic factor for both OS and PFS in glioma patients. Moreover, the effects of RTL on the prognosis of patients exhibited a dose-dependent manner. Stratified analysis showed that the prognostic value of RTL was not affected by host characteristics except for age. In addition, flow cytometry and ELISA analyses indicated that there was no significant association between RTL and frequency of different immune cell subsets or plasma cytokine concentrations.
Conclusions: Our study for the first time demonstrates that leukocyte RTL is an independent prognostic marker for glioma patients. The potential mechanism needs further investigation.
Conflict of interest statement
Authors declare that there is no competing interest.
Figures
References
-
- Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, DePinho RA (2000) Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 406(6796):641–645. doi:10.1038/35020592 - PubMed
-
- Baydar DE, Ozen H, Geyik PO, Gurel B (2010) Can telomere alterations predict biochemical recurrence in prostate adenocarcinoma? A preliminary study. Pathol Res Pract 206(10):700–704. doi:10.1016/j.prp.2010.05.009 - PubMed
-
- Bekaert S, De Meyer T, Van Oostveldt P (2005) Telomere attrition as ageing biomarker. Anticancer Res 25(4):3011–3021 - PubMed
-
- Blasco MA (2007) The epigenetic regulation of mammalian telomeres. Nat Rev Genet 8(4):299–309. doi:10.1038/nrg2047 - PubMed
-
- Brandsma D, van den Bent MJ (2009) Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol 22(6):633–638. doi:10.1097/WCO.0b013e328332363e - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

