The neuropathology of traumatic brain injury
- PMID: 25702209
- PMCID: PMC4694720
- DOI: 10.1016/B978-0-444-52892-6.00004-0
The neuropathology of traumatic brain injury
Abstract
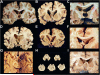
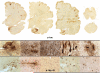
Traumatic brain injury, a leading cause of mortality and morbidity, is divided into three grades of severity: mild, moderate, and severe, based on the Glasgow Coma Scale, the loss of consciousness, and the development of post-traumatic amnesia. Although mild traumatic brain injury, including concussion and subconcussion, is by far the most common, it is also the most difficult to diagnose and the least well understood. Proper recognition, management, and treatment of acute concussion and mild traumatic brain injury are the fundamentals of an emerging clinical discipline. It is also becoming increasingly clear that some mild traumatic brain injuries have persistent, and sometimes progressive, long-term debilitating effects. Evidence indicates that a single traumatic brain injury can precipitate or accelerate multiple age-related neurodegenerations, increase the risk of developing Alzheimer's disease, Parkinson's disease, and motor neuron disease, and that repetitive mild traumatic brain injuries can provoke the development of a tauopathy, chronic traumatic encephalopathy. Clinically, chronic traumatic encephalopathy is associated with behavioral changes, executive dysfunction, memory loss, and cognitive impairments that begin insidiously and progress slowly over decades. Pathologically, chronic traumatic encephalopathy produces atrophy of the frontal and temporal lobes, thalamus, and hypothalamus, septal abnormalities, and abnormal deposits of hyperphosphorylated tau (τ) as neurofibrillary tangles and disordered neurites throughout the brain. The incidence and prevalence of chronic traumatic encephalopathy and the genetic risk factors critical to its development are currently unknown. Chronic traumatic encephalopathy frequently occurs as a sole diagnosis, but may be associated with other neurodegenerative disorders, including Alzheimer's disease, Lewy body disease, and motor neuron disease. Currently, chronic traumatic encephalopathy can be diagnosed only at autopsy; however, promising efforts to develop imaging, spinal fluid, and peripheral blood biomarkers are underway to diagnose and monitor the course of disease in living subjects.
Keywords: Axonal injury; blast injury; brain trauma; concussion; mild traumatic brain injury; motor neuron disease; post-traumatic neurodegeneration; tau protein.
© 2015 Elsevier B.V. All rights reserved.
Figures



References
-
- Baker M, Litvan I, Houlden H, et al. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum Mol Genet. 1999;8:711–715. - PubMed
-
- Baugh CM, Stamm JM, Riley DO, et al. Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav. 2012;6:244–254. - PubMed
-
- Belli S, Vanacore N. Proportionate mortality of Italian soccer players: is amyotrophic lateral sclerosis an occupational disease? Eur J Epidemiol. 2005;20:237–242. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

