Neuroprotection for traumatic brain injury
- PMID: 25702227
- PMCID: PMC4808298
- DOI: 10.1016/B978-0-444-52892-6.00022-2
Neuroprotection for traumatic brain injury
Abstract
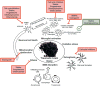
Traumatic brain injury (TBI) is a major cause of mortality and morbidity worldwide. Despite extensive preclinical research supporting the effectiveness of neuroprotective therapies for brain trauma, there have been no successful randomized controlled clinical trials to date. TBI results in delayed secondary tissue injury due to neurochemical, metabolic and cellular changes; modulating such effects has provided the basis for neuroprotective interventions. To establish more effective neuroprotective treatments for TBI it is essential to better understand the complex cellular and molecular events that contribute to secondary injury. Here we critically review relevant research related to causes and modulation of delayed tissue damage, with particular emphasis on cell death mechanisms and post-traumatic neuroinflammation. We discuss the concept of utilizing multipotential drugs that target multiple secondary injury pathways, rather than more specific "laser"-targeted strategies that have uniformly failed in clinical trials. Moreover, we assess data supporting use of neuroprotective drugs that are currently being evaluated in human clinical trials for TBI, as well as promising emerging experimental multipotential drug treatment strategies. Finally, we describe key challenges and provide suggestions to improve the likelihood of successful clinical translation.
Keywords: Traumatic brain injury; cell death mechanisms; inflammation; multipotential drugs; neuroprotection.
© 2015 Elsevier B.V. All rights reserved.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources