Inherited CARD9 deficiency in otherwise healthy children and adults with Candida species-induced meningoencephalitis, colitis, or both
- PMID: 25702837
- PMCID: PMC4831587
- DOI: 10.1016/j.jaci.2014.12.1930
Inherited CARD9 deficiency in otherwise healthy children and adults with Candida species-induced meningoencephalitis, colitis, or both
Abstract
Background: Invasive infections of the central nervous system (CNS) or digestive tract caused by commensal fungi of the genus Candida are rare and life-threatening. The known risk factors include acquired and inherited immunodeficiencies, with patients often displaying a history of multiple infections. Cases of meningoencephalitis, colitis, or both caused by Candida species remain unexplained.
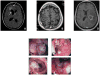
Objective: We studied 5 previously healthy children and adults with unexplained invasive disease of the CNS, digestive tract, or both caused by Candida species. The patients were aged 39, 7, 17, 37, and 26 years at the time of infection and were unrelated, but each was born to consanguineous parents of Turkish (2 patients), Iranian, Moroccan, or Pakistani origin. Meningoencephalitis was reported in 3 patients, meningoencephalitis associated with colitis was reported in a fourth patient, and the fifth patient had colitis only.
Methods: Inherited caspase recruitment domain family, member 9 (CARD9) deficiency was recently reported in otherwise healthy patients with other forms of severe disease caused by Candida, Trichophyton, Phialophora, and Exophiala species, including meningoencephalitis but not colitis caused by Candida and Exophiala species. Therefore we sequenced CARD9 in the 5 patients.
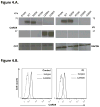
Results: All patients were found to be homozygous for rare and deleterious mutant CARD9 alleles: R70W and Q289* for the 3 patients with Candida albicans-induced meningoencephalitis, R35Q for the patient with meningoencephalitis and colitis caused by Candida glabrata, and Q295* for the patient with Candida albicans-induced colitis. Regardless of their levels of mutant CARD9 protein, the patients' monocyte-derived dendritic cells responded poorly to CARD9-dependent fungal agonists (curdlan, heat-killed C albicans, Saccharomyces cerevisiae, and Exophiala dermatitidis).
Conclusion: Invasive infections of the CNS or digestive tract caused by Candida species in previously healthy children and even adults might be caused by inherited CARD9 deficiency.
Keywords: Candida species; Inborn error of immunity; central nervous system; colitis; human; inherited CARD9 deficiency; invasive fungal diseases; primary immunodeficiency.
Copyright © 2015 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures





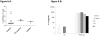
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

