Activation of β2-adrenergic receptor by (R,R')-4'-methoxy-1-naphthylfenoterol inhibits proliferation and motility of melanoma cells
- PMID: 25703025
- PMCID: PMC4361792
- DOI: 10.1016/j.cellsig.2015.02.012
Activation of β2-adrenergic receptor by (R,R')-4'-methoxy-1-naphthylfenoterol inhibits proliferation and motility of melanoma cells
Abstract
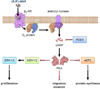
(R,R')-4'-methoxy-1-naphthylfenoterol [(R,R')-MNF] is a highly-selective β2 adrenergic receptor (β2-AR) agonist. Incubation of a panel of human-derived melanoma cell lines with (R,R')-MNF resulted in a dose- and time-dependent inhibition of motility as assessed by in vitro wound healing and xCELLigence migration and invasion assays. Activity of (R,R')-MNF positively correlated with the β2-AR expression levels across tested cell lines. The anti-motility activity of (R,R')-MNF was inhibited by the β2-AR antagonist ICI-118,551 and the protein kinase A inhibitor H-89. The adenylyl cyclase activator forskolin and the phosphodiesterase 4 inhibitor Ro 20-1724 mimicked the ability of (R,R')-MNF to inhibit migration of melanoma cell lines in culture, highlighting the importance of cAMP for this phenomenon. (R,R')-MNF caused significant inhibition of cell growth in β2-AR-expressing cells as monitored by radiolabeled thymidine incorporation and xCELLigence system. The MEK/ERK cascade functions in cellular proliferation, and constitutive phosphorylation of MEK and ERK at their active sites was significantly reduced upon β2-AR activation with (R,R')-MNF. Protein synthesis was inhibited concomitantly both with increased eEF2 phosphorylation and lower expression of tumor cell regulators, EGF receptors, cyclin A and MMP-9. Taken together, these results identified β2-AR as a novel potential target for melanoma management, and (R,R')-MNF as an efficient trigger of anti-tumorigenic cAMP/PKA-dependent signaling in β2-AR-expressing lesions.
Keywords: Beta blocker; Beta2 adrenoreceptor selective agonist; Melanocortin 1 receptor; Melanocyte; Metastasis; Migration.
Published by Elsevier Inc.
Conflict of interest statement
The authors state no conflict of interest other than the fact that IW Wainer, M Bernier, RK Paul, LR Toll and LA Jimenez are listed as co-inventors on a patent application for the use of fenoterol and other fenoterol derivatives [including (
Figures






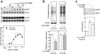
Similar articles
-
Concurrent activation of β2-adrenergic receptor and blockage of GPR55 disrupts pro-oncogenic signaling in glioma cells.Cell Signal. 2017 Aug;36:176-188. doi: 10.1016/j.cellsig.2017.05.006. Epub 2017 May 8. Cell Signal. 2017. PMID: 28495590 Free PMC article.
-
Cannabinoid receptor activation correlates with the proapoptotic action of the β2-adrenergic agonist (R,R')-4-methoxy-1-naphthylfenoterol in HepG2 hepatocarcinoma cells.J Pharmacol Exp Ther. 2012 Oct;343(1):157-66. doi: 10.1124/jpet.112.195206. Epub 2012 Jul 9. J Pharmacol Exp Ther. 2012. PMID: 22776956 Free PMC article.
-
Heterodimerization With 5-HT2BR Is Indispensable for β2AR-Mediated Cardioprotection.Circ Res. 2021 Jan 22;128(2):262-277. doi: 10.1161/CIRCRESAHA.120.317011. Epub 2020 Nov 19. Circ Res. 2021. PMID: 33208036
-
Effect of fenoterol stereochemistry on the β2 adrenergic receptor system: ligand-directed chiral recognition.Chirality. 2011;23 Suppl 1(Suppl 1):E1-6. doi: 10.1002/chir.20963. Epub 2011 May 26. Chirality. 2011. PMID: 21618615 Free PMC article. Review.
-
Activation of β2 adrenergic receptor signaling modulates inflammation: a target limiting the progression of kidney diseases.Arch Pharm Res. 2021 Jan;44(1):49-62. doi: 10.1007/s12272-020-01280-9. Epub 2020 Nov 5. Arch Pharm Res. 2021. PMID: 33155167 Review.
Cited by
-
Elongation factor 2 in cancer: a promising therapeutic target in protein translation.Cell Mol Biol Lett. 2024 Dec 20;29(1):156. doi: 10.1186/s11658-024-00674-7. Cell Mol Biol Lett. 2024. PMID: 39707196 Free PMC article. Review.
-
The beta adrenergic receptor antagonist propranolol alters mitogenic and apoptotic signaling in late stage breast cancer.Biomed J. 2019 Jun;42(3):155-165. doi: 10.1016/j.bj.2019.02.003. Epub 2019 Jul 25. Biomed J. 2019. PMID: 31466709 Free PMC article.
-
Structural Insights into Ligand-Receptor Interactions Involved in Biased Agonism of G-Protein Coupled Receptors.Molecules. 2021 Feb 6;26(4):851. doi: 10.3390/molecules26040851. Molecules. 2021. PMID: 33561962 Free PMC article. Review.
-
Activation of β2-adrenergic receptor signals suppresses mesenchymal phenotypes of oral squamous cell carcinoma cells.Cancer Sci. 2021 Jan;112(1):155-167. doi: 10.1111/cas.14670. Epub 2020 Nov 18. Cancer Sci. 2021. PMID: 33007125 Free PMC article.
-
Deprogramming metabolism in pancreatic cancer with a bi-functional GPR55 inhibitor and biased β2 adrenergic agonist.Sci Rep. 2022 Mar 7;12(1):3618. doi: 10.1038/s41598-022-07600-x. Sci Rep. 2022. PMID: 35256673 Free PMC article.
References
-
- Yang G, Zhang G, Pittelkow MR, Ramoni M, Tsao H. J Invest Dermatol. 2006;126:2490–2506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

