Macrophage defense mechanisms against intracellular bacteria
- PMID: 25703560
- PMCID: PMC4368383
- DOI: 10.1111/imr.12266
Macrophage defense mechanisms against intracellular bacteria
Abstract
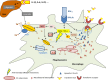
Macrophages and neutrophils play a decisive role in host responses to intracellular bacteria including the agent of tuberculosis (TB), Mycobacterium tuberculosis as they represent the forefront of innate immune defense against bacterial invaders. At the same time, these phagocytes are also primary targets of intracellular bacteria to be abused as host cells. Their efficacy to contain and eliminate intracellular M. tuberculosis decides whether a patient initially becomes infected or not. However, when the infection becomes chronic or even latent (as in the case of TB) despite development of specific immune activation, phagocytes have also important effector functions. Macrophages have evolved a myriad of defense strategies to combat infection with intracellular bacteria such as M. tuberculosis. These include induction of toxic anti-microbial effectors such as nitric oxide and reactive oxygen intermediates, the stimulation of microbe intoxication mechanisms via acidification or metal accumulation in the phagolysosome, the restriction of the microbe's access to essential nutrients such as iron, fatty acids, or amino acids, the production of anti-microbial peptides and cytokines, along with induction of autophagy and efferocytosis to eliminate the pathogen. On the other hand, M. tuberculosis, as a prime example of a well-adapted facultative intracellular bacterium, has learned during evolution to counter-balance the host's immune defense strategies to secure survival or multiplication within this otherwise hostile environment. This review provides an overview of innate immune defense of macrophages directed against intracellular bacteria with a focus on M. tuberculosis. Gaining more insights and knowledge into this complex network of host-pathogen interaction will identify novel target sites of intervention to successfully clear infection at a time of rapidly emerging multi-resistance of M. tuberculosis against conventional antibiotics.
Keywords: efferocytosis; iron; macrophages; phagolysosome; radicals; vitamin D.
© 2015 The Authors. Immunological Reviews published by John Wiley & Sons Ltd.
Figures

References
-
- Bruns H, Stenger S. New insights into the interaction of Mycobacterium tuberculosis and human macrophages. Future Microbiol. 2014;9:327–341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

