Mycobacteria, metals, and the macrophage
- PMID: 25703564
- PMCID: PMC4521620
- DOI: 10.1111/imr.12265
Mycobacteria, metals, and the macrophage
Abstract
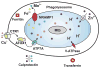
Mycobacterium tuberculosis is a facultative intracellular pathogen that thrives inside host macrophages. A key trait of M. tuberculosis is to exploit and manipulate metal cation trafficking inside infected macrophages to ensure survival and replication inside the phagosome. Here, we describe the recent fascinating discoveries that the mammalian immune system responds to infections with M. tuberculosis by overloading the phagosome with copper and zinc, two metals which are essential nutrients in small quantities but are toxic in excess. M. tuberculosis has developed multi-faceted resistance mechanisms to protect itself from metal toxicity including control of uptake, sequestration inside the cell, oxidation, and efflux. The host response to infections combines this metal poisoning strategy with nutritional immunity mechanisms that deprive M. tuberculosis from metals such as iron and manganese to prevent bacterial replication. Both immune mechanisms rely on the translocation of metal transporter proteins to the phagosomal membrane during the maturation process of the phagosome. This review summarizes these recent findings and discusses how metal-targeted approaches might complement existing TB chemotherapeutic regimens with novel anti-infective therapies.
Keywords: copper; innate immunity; iron; manganese; nutritional immunity; phagosome; poisoning; zinc.
© 2015 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
References
-
- van der Wel N, et al. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell. 2007;129:1287–1298. - PubMed
-
- Welin A, Lerm M. Inside or outside the phagosome? The controversy of the intracellular localization of Mycobacterium tuberculosis. Tuberculosis (Edinb) 2012;92:113–120. - PubMed
-
- Vergne I, Chua J, Singh SB, Deretic V. Cell biology of Mycobacterium tuberculosis phagosome. Annu Rev Cell Dev Biol. 2004;20:367–394. - PubMed
-
- Rohde K, Yates RM, Purdy GE, Russell DG. Mycobacterium tuberculosis and the environment within the phagosome. Immunol Rev. 2007;219:37–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

